Nanofludic channels offer new opportunities for biotechnology and medicine, including separation of biomolecules, drug delivery, and single molecule detection because they provide unique capability. In addition to various applications, nanofluidic channel can be an ideal, well-controlled experimental platform to study nanoscale molecular, fluidic, or ionic transport properties. However, the fabrication of nanofluidic channels aligned with microfluidic networks currently requires precise lithography and nanofabrication procedures. To address the need for a simple, low-cost fabrication method for forming close-packed nanofluidic channels, we explore various approaches; a) Advanced conventional MEMS methods: fabrication process for planar nanochannel system and thermal oxide growth process for vertical nanochannel system; b) Non-lithographical methods: junction gap breakdown, wrinkles, surface patterning, and self-sealed nanoporous junctions.
• Jonathan P. DeRocher, Pan Mao, Jongyoon Han, Michael F. Rubner and Robert E. Cohen, “Layer-by-Layer Assembly of Polyelectrolytes in Nanofluidic Devices,” Macromolecules, 2010, 43(5), 2430-2437.
A hybrid micro-/nanofluidic device which contains an array of parallel nanochannels has been employed to study polyelectrolyte multilayer (PEM) deposition in confined geometries. Layer-by-layer (LbL) assembly of poly(allylamine hydrochloride) (PAH) and poly(styrenesulfonate) (PSS) at pH 4 and salt concentrations ranging from 0.1 to 1 M was used to conformally coat the nanochannel walls, systematically narrowing the channel width from 222 to 11 nm in the wet state. The thicknesses of confined multilayers were measured using SEM and these results were compared with those obtained on planar, unconfined surfaces. A procedure for direct measurement of the gap thickness using dc conductance was also developed. LbL assembly in the nanochannels resulted in lower bilayer thicknesses than those obtained on planar surfaces. This observation is attributed to the surface charge-induced depletion of unadsorbed polyelectrolytes within the channel. The ability to conformally coat the walls of the nanochannels with functional PEMs opens up new possibilities in the design of active nanochannel devices.
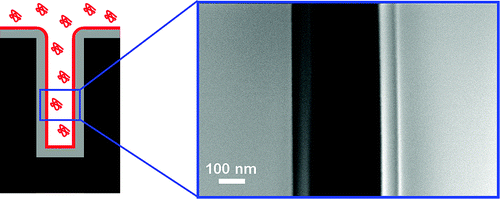
• Mao, P. and Han, J., “Massively-Parallel Ultra-High-Aspect-Ratio Nanochannels as Mesoporous Membranes,” Lab on a Chip, 9, 586-591 (2009).
We develop a novel fabrication strategy for generating massively-parallel, regular vertical nanochannel membranes with a uniform, well-controlled gap size of ~50 nm and a depth up to ~40 ㎛, by using only standard semiconductor fabrication techniques. A continuous-flow separation of large DNAs and small molecules is demonstrated with large open volume, enabling high-throughput applications.
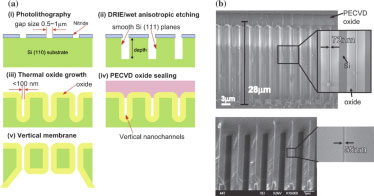
• Chung, S., Lee, J. H., Moon, M-W., Han, J and Kamm R. D. “Non-lithographic wrinkle nanochannels for protein preconcentration,” Advanced Matererials, 20, 3011-3016 (2008).
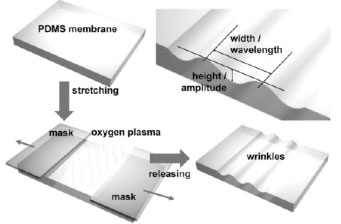
This paper explored an approach that produces shallow wrinkles on a poly (dimethylsiloxane) (PDMS) substrate. Wrinkle pattern could be created by stretching a sheet of PDMS and then treating the surface with oxygen plasma to generate a stiff skin. Upon releasing the pre-stress, the wrinkle nanochannels (WNC) are formed. WNCs can easily form uniform arrays of high density with controllable dimensions in only a specific region. WNCs are useful for protein preconcentration, which varies linearly in time. Preconcentration levels of more than 100 can be achieved within 10 minutes.
• Yong-Ak Song, Candy Batista, Raul Sarpeshkar and Jongyoon Han, “Rapid fabrication of microfluidic polymer electrolyte membrane fuel cell in PDMS by surface patterning of perfluorinated ion-exchange resin,” Journal of Power Sources, 183, 674-677, 2008.**
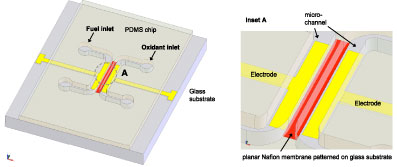
In this paper, we demonstrate a simple and rapid fabrication method of microfluidic polymer electrolyte membrane (PEM) fuel cell in polydimethylsiloxane (PDMS), which has become de facto standard material in BioMEMS. Instead of integrating a Nafion sheet film between two layers of the PDMS device in the “sandwich format”, we pattern perfluorinated ion-exchange resin such as Nafion resin on a glass substrate using a reversibly bonded PDMS microchannel and generate a submicron thin ion-selective membrane between the electrodes. After pattering of the membrane, the assembly of the fuel cell is accomplished simply by standard oxygen plasma bonding between the PDMS chip and the glass substrate. The planar PEM microfluidic fuel cell can generate an open circuit voltage between 600-800mV and deliver a maximum current output of 4 μA. In order to enhance the power output, we utilize the self-assembled silica colloidal arrays as a support matrix for Nafion resin. In this way, we can increase the thickness of the ion-selective membrane up to 20 μm and the current density by 166%. This new fabrication method enables a rapid prototyping of microfluidic fuel cells to study alternative ion-exchange resins for the polymer electrolyte membrane and opens up the possibility of miniature, implantable on-chip power sources for biomedicalapplications.
• Lee, J. H., Song, Y.-A., and Han, J. “Multiplexed Proteomic Sample Preconcentration Device Using Surface-Patterned Ion-Selective Membrane,” Lab on a Chip, 8, 596-601 (2008).
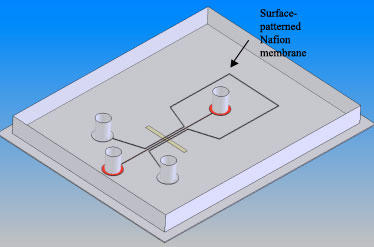
A high-throughput protein preconcentrator in poly (dimethylsiloxane) (PDMS) is reported by using a surface-patterned ion-selective membrane. A thin printed Nafion membrane is integrated by plasma bonding between the PDMS chip and the glass substrate. The PDMS preconcentrator shows a concentration factor as high as 104 in 5 min. This printing method also allows fabricating a parallel array of preconcentrators to increase the concentrated sample volume.
• Kim, S. J. and Han, J. “Self-Sealed Vertical Polymeric Nanoporous-Junctions for High-Throughput Nanofluidic Applications,” Analytical Chemistry, 80, 3507-3511 (2008).
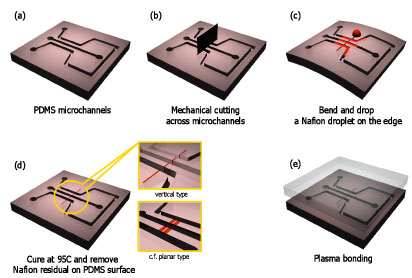
A novel fabrication method is presented for polymeric nanoporous-junctions integrated inside poly(dimethylsiloxane) (PDMS) microchips. The nanoporous junction was created by infiltrating polymer solution between the gaps created simply by mechanical cutting, without cleanroom processes. The PDMS can seal itself with the heterogeneous nanoporous material between PDMS/PDMS gap, due to its flexibility without any (covalent) chemical bonding between PDMS and porous materials. This allows one to integrate nanoporous-junction into PDMS microchip in a leak-free manner with excellent repeatability. In addition, vertical nanojunctions are better coupled with the microchannel, allows efficient operations.High throughput nanofluidic preconcentrator in a large microchannel (1000 μm width × 100 μm depth) was successfully demonstrated, which was enabled by the efficient vertical integration between nanoporous-junctions and microchannels.
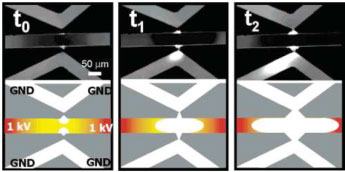
• Lee, J. H., Chung, S., Kim, S. J. and Han, J. “Novel PDMS based protein preconcentration using a nanogap generated by junction gap breakdown,” Analytical Chemistry, 79, 6868 -6873 (2007).
A nanofluidic preconcentrator on a poly(dimethylsiloxane) (PDMS)-based microfluidic channel is developed by simple nanogap formation using the junction gap breakdown phenomenon between two PDMS microchannels. The concentration volume of β-phycoerythrin protein as high as 70 pL is achieved with a concentration factor up to 104 within 1 h. The availability of protein preconcentration under several different buffers (phosphate, acetate) at several different pH values (pH 5 to pH 9) is also demonstrated.
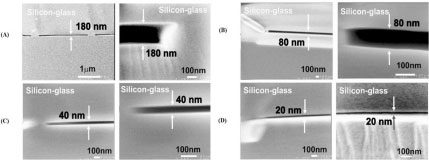
• Mao, P. and Han, J. “Fabrication and characterization of 20 nm nanofluidic channels by glass-glass and glass-silicon bonding,” Lab on a Chip 5, 837-844 (2005).
We have characterized glass-glass and glass-Si bonding processes for the fabrication of wide, shallow nanofluidic channels as thin as 20nm with large uniformity. The aspect ratio of the planar nanochannels achieved (0.004~0.0002) is small enough, so that standard photolithography can be used to generate nanofluidic channels. This results will be useful in designing next-generation nanofluidic devices that can be used for protein separation and biomolecule preconcentration.