News
How ultracold, superdense atoms become invisible
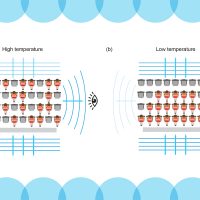
An atom’s electrons are arranged in energy shells. Like concertgoers in an arena, each electron occupies a single chair and cannot drop to a lower tier if all its chairs are occupied. This fundamental property of atomic physics is known as the Pauli exclusion principle, and it explains the shell structure of atoms, the diversity of the periodic table of elements, and the stability of the material universe.
Now, MIT physicists have observed the Pauli exclusion principle, or Pauli blocking, in a completely new way: They’ve found that the effect can suppress how a cloud of atoms scatters light.