News
Uncovering the mystery of the disappearing molecules
Ultracold molecules are a research frontier that builds upon the many successes of ultracold atoms in the study of quantum science. There has been much experimental effort dedicated to the realization of trapped gaseous samples of ultracold molecules. In fact, various research groups from around the world have successfully demonstrated methods to bring samples of diatomic molecules (e.g. KRb, NaK, NaRb, and RbCs) into the quantum regime. Upon doing so, however, it was observed, to the surprise of many, that the molecules in these systems were quickly lost, regardless of the specific molecular species that was studied. The cause of this loss remained a mystery for nearly a decade, until theoretical work from CUA members Tijs Karman and Martin Zwierlein, combined with experimental work from a group led by CUA member Kang-Kuen Ni at Harvard University uncovered the answer.
While previous experiments could only detect a single molecular species in specific internal states, the team at Harvard developed a more general detection technique that provided new insight into the problem. Borrowing methods from the field of physical chemistry, the researchers were able to ionize different molecular species within the sample with intense pulses of ultraviolet light, and to measure these ions using electric fields that accelerate them onto an ion detector regardless of the molecular internal states.
Applying this detection method to an ultracold gas of KRb molecules, the team observed the presence of K2 and Rb2 in the sample, which are the result of exothermic chemical reactions between KRb molecules, as well as K2Rb2, a typically elusive intermediate molecular complex formed when two KRb molecules collide. The presence of chemical reactions in this system, however, could not explain the mysterious loss observed in other experiments on different molecular species (e.g. NaK, NaRb, or RbCs), where reactions are energetically forbidden. What is common among the different experiments though is the existence of an intermediate complex, and the use of intense beams of infrared (1064 nm) laser light, as these lasers provide a technologically simple means to trap and confine the molecular samples. This commonality prompted the hypothesis that the light could be the source of the observed loss, as it may photo-excite any long-lived complexes that are formed when molecules collide.
To investigate this, the Harvard researchers examined the effect of 1064 nm laser light on both the K2Rb2 intermediate complex and the K2 and Rb2 reaction products. What they observed is that increasing the intensity of the light decreased the concentration of K2Rb2 complexes. In fact, at sufficiently high laser intensity, it was discovered that the entire K2Rb2 population could be depleted. At the same time, the researchers found that the K2 and Rb2 product populations declined with the decreasing K2Rb2 population, a sign that the laser light was photo-exciting the molecular complex and expelling it from the sample, thereby suppressing product formation.
Taking advantage of this effect, the researchers were able to measure the amount of time that the system spends in the transition complex before it dissociates into products by monitoring the dynamics of the K2Rb2 population after a sudden turn-off of the 1064 nm light. The measured lifetime of 360 ns is orders of magnitude longer than that of most intermediate complexes involved in higher temperature chemical reactions. These observations directly demonstrate that complexes formed through collisions of ultracold molecules live long enough to make them extremely susceptible to photo-excitation by the laser light used to confine them, thus resolving the mystery of why even non-reactive molecular systems experience loss when trapped by tightly focused lasers.
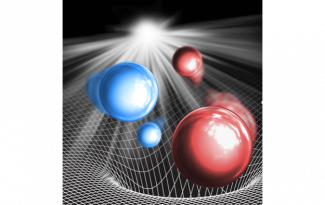
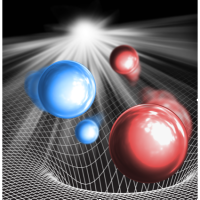
Image Caption: A schematic depiction of K (red) and Rb (blue) atoms within the K2Rb2 intermediate complex that is formed through collisions of ultracold KRb molecules. The grid sitting underneath the complex represents the potential energy surface of the KRb + KRb system, where the deep well illustrates the significant binding energy associated with the lowest energy configurations of the complex. The light that is shown illuminating the system represents the 1064 nm laser used to photo-excite the complex. (Image credit: Yu Liu)