The vital role of electrolyte in beyond Li-ion batteries
(Credit: Graham Leverick)
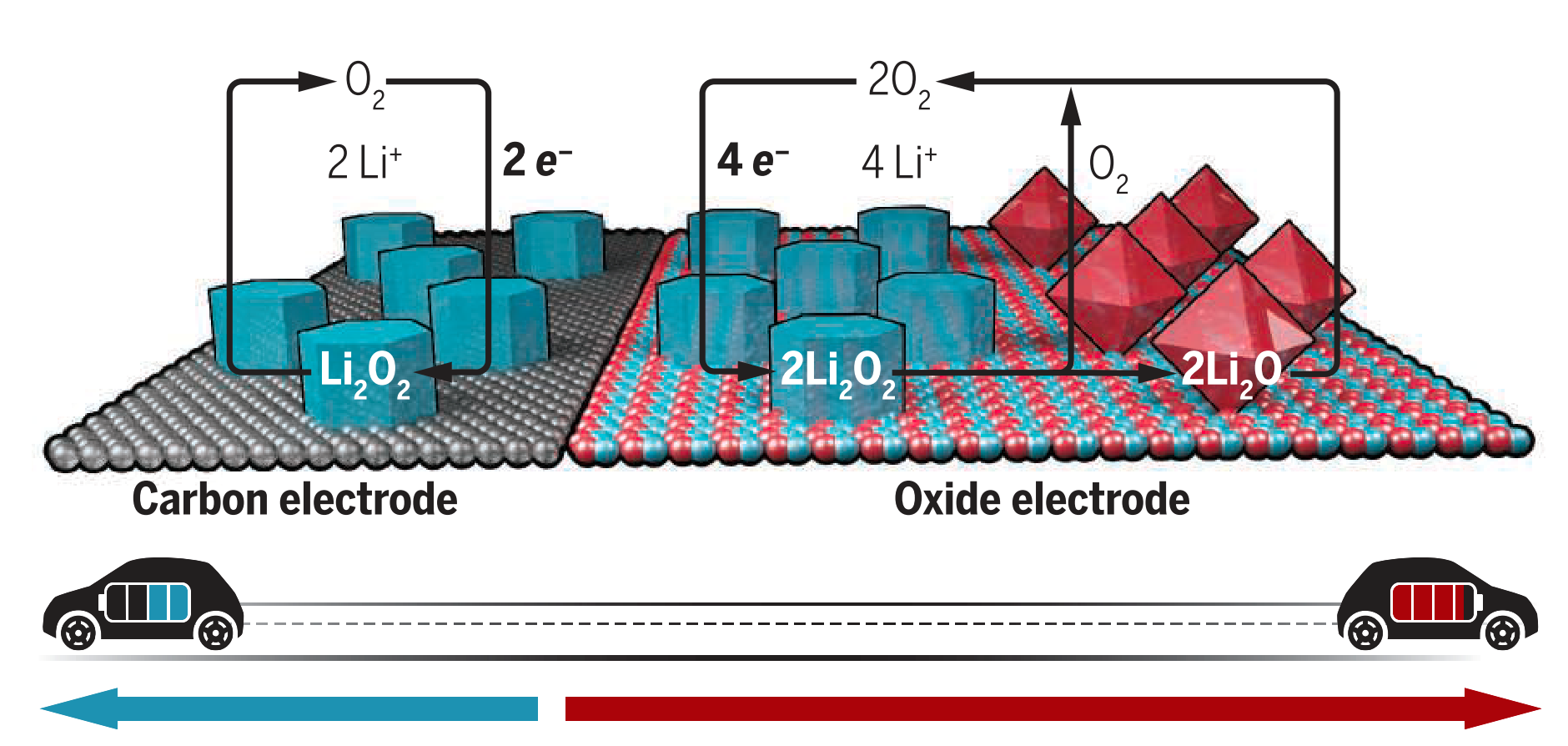
While the modern Li-ion battery is a scientific and engineering marvel, having enabled substantial technological innovation spanning from mobile electronics to commercially viable electric vehicles, the intercalation chemistry of the Li-ion battery has a fundamental upper limit on its achievable energy density owing to the fact that the same structure must remain stable in the fully lithiated and delithiated states. While further advances within the Li-ion chemistry are possible, significantly higher theoretical energy densities are achievable with conversion chemistries, such as Li-O2 and Li-S batteries1, where the structure and/or phase of the electrode materials change with its state of charge. While the importance of electrolyte electrochemical/chemical stability and ionic conductivity has been known since the very first batteries, owing to the partial solubility of reaction intermediates in conversion chemistries (such as Li+-O2– and Li2Sx), electrolyte can play an even more critical role in determining their reaction pathway and kinetics.
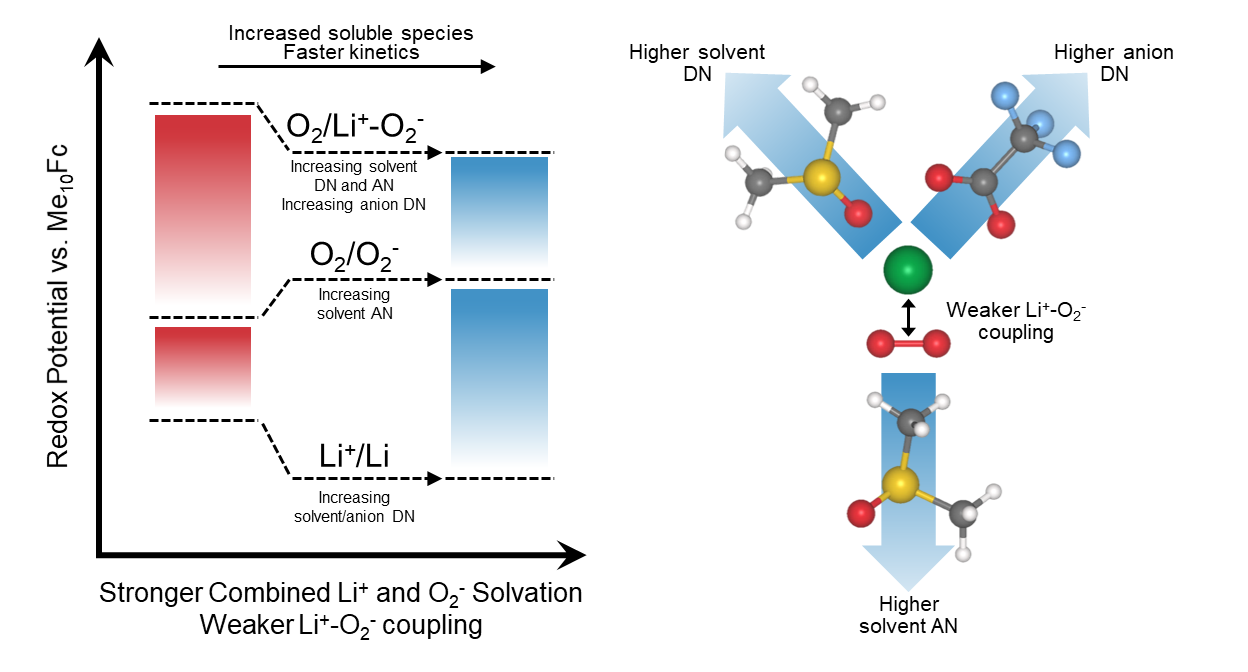
Through detailed mechanistic studies on the discharge process in Li-O2 batteries, our research has uncovered that that the role of solvent, counter anion and salt concentration can all be understood based on their influence on the Li+-O2– coupling strength. We show that Li+-O2– coupling strength is governed by the combined solvation energy of Li+ and O2– ions, where stronger combined solvation energy promotes the dissociation reaction given by Li+-O2– → Li+ + O2–. This allows us to rationally design the electrolyte (solvent + salt + salt concentration) in Li-O2 batteries to control the reaction pathway and kinetics.[Key references: Leverick et al.2 Kwabi et al.3]
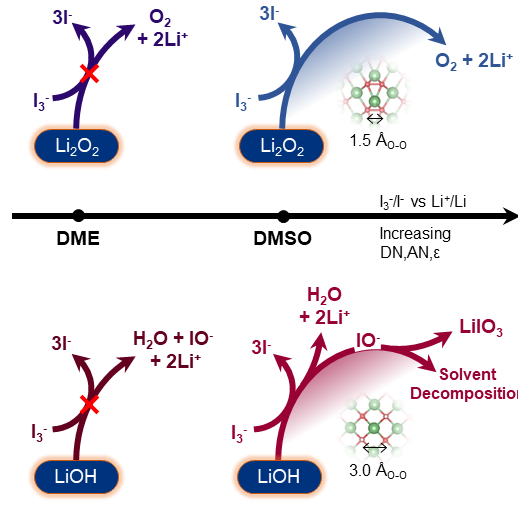
Our work in electrolytes for Li-O2 batteries has also shown the synergistic interactions between electrolyte and soluble redox mediators. Soluble redox mediators enhance the kinetics of Li-O2 reactions by promoting electron transfer to the surface of the electronically insulating Li2O2, where the redox mediator is first electrochemically oxidized at the electrode surface and then the oxidized form of the redox mediator chemically oxidizes Li2O2 to form Li+ ions and molecular oxygen and regenerate the reduced form of the redox mediator. Since this reaction takes place in solution, the thermodynamics and kinetics of the redox mediator reactions can be significantly altered through solvation, where stronger solvation of Li+ ions and the reduced form of the redox mediator can help drive the reaction forwards, enhancing Li2O2 oxidation. Interactions between the solvent, additives like water, and the redox mediator can also influence the reaction pathway, leading to parasitic reactions.
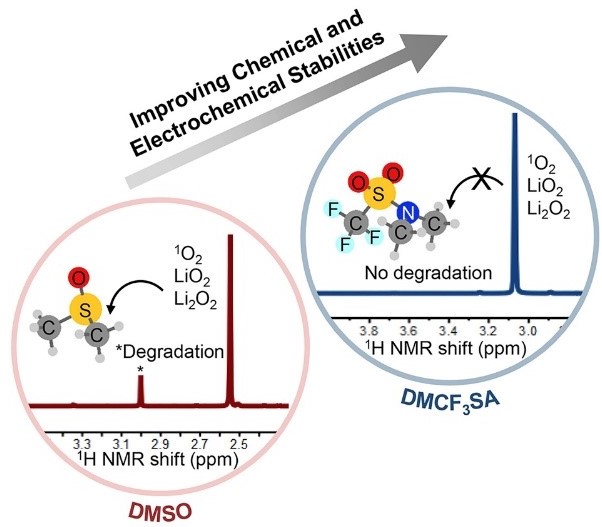
Finally, our research has also focused on developing new electrolytes that can withstand the harsh environment of the Li-O2 battery. We first derived a systematic chemical framework with which to assess what chemical structures were stable against decompositions from hydrogen abstract, deprotonation, nucleophilic attack, electrochemical oxidation and reactions with singlet oxygen6. Based on these design criteria, we were able to design new solvents which are resistant to decomposition7 and demonstrate great stability in Li-O2 batteries.[Key references: Feng et al.7 Feng et al.6]
References:
1. Bruce, P. G., Freunberger, S. A., Hardwick, L. J., Tarascon, J.-M. Li–O2 and Li–S Batteries with High Energy Storage. Nat. Mater., 11 (1), 19–29 (2011).
2. Leverick, G., Tatara, R., Feng, S., Crabb, E., France-Lanord, A., Tulodziecki, M., Lopez, J., Stephens, R., Grossman, J. C., Shao-Horn, Y. Solvent- and Anion-Dependent Li+-O2– Coupling Strength and Implications on the Thermodynamics and Kinetics of Li-O 2 Batteries. J. Phys. Chem. C, 124 (9), 4953–4967 (2020).
3. Kwabi, D. G., Bryantsev, V. S., Batcho, T. P., Itkis, D. M., Thompson, C. V., Shao-Horn, Y. Experimental and Computational Analysis of the Solvent-Dependent O2/Li +-O2− Redox Couple: Standard Potentials, Coupling Strength, and Implications for Lithium-Oxygen Batteries. Angew. Chem. Int. Ed. 55 (9), 3129–3134 (2016).
4. Leverick, G., Tułodziecki, M., Tatara, R., Barde´, F., Shao-Horn, Y. Solvent-Dependent Oxidizing Power of LiI Redox Couples for Li-O2 Batteries. Joule, 3, 1–21 (2019).
5. Tułodziecki, M., Leverick, G. M., Amanchukwu, C. V., Katayama, Y., Kwabi, D. G., Bardé, F., Hammond, P. T., Shao-Horn, Y. The Role of Iodide in the Formation of Lithium Hydroxide in Lithium–Oxygen Batteries. Energy Env. Sci, 10 (8), 1828–1842 (2017).
6. Feng, S., Chen, M., Giordano, L., Huang, M., Zhang, W., Amanchukwu, C. V., Anandakathir, R., Shao-Horn, Y., Johnson, J. A. Mapping a Stable Solvent Structure Landscape for Aprotic Li–Air Battery Organic Electrolytes. J. Mater. Chem. A, 5 (45), 23987–23998 (2017).
7. Feng, S., Huang, M., Lamb, J. R., Zhang, W., Tatara, R., Zhang, Y., Guang Zhu, Y., Perkinson, C. F., Johnson, J. A., Shao-Horn, Y. Molecular Design of Stable Sulfamide- and Sulfonamide-Based Electrolytes for Aprotic Li-O2 Batteries. Chem, 5 (10), 2630-2641 (2019).
Semi-solid flow battery
(Credit: Thaneer Malai Narayanan)
Cost efficient energy storage system is needed for deep decarbonization of the power and transportation sector. Common battery electrode design such as in Li-ion, is to deposit the redox material powder on a conductive substrate, limits the power and energy output to fixed ratio, preventing it to achieve low-cost energy storage system. Semi-solid flowable electrode overcomes this limitation by suspending redox material powder in a liquid electrolyte.1 The electrically and ionically conductive, and flowable nature of the semi-solid electrode allows solid active materials to be used in energy and power decoupled flow battery systems (semi-solid flow battery, SSFB). In this research, we are exploring the design criteria to develop battery prototypes with semi-solid electrodes using electrochemical and rheological experiments and techno-economic modelling.
Semi-solid electrode consists of active material powder (e.g. Ni(OH)2, MnO2, LiCoO2, sulfur), conductive additive (e.g. carbon black, metal powders), polymer as interparticle force modifier, and electrolyte. Composition of semi-solid electrode heavily influences the mechanical and electrochemical properties of the battery. For example, interparticle attraction of carbon black particles can increase the minimum macroscopic stress required for semi-solid electrode to yield during flow.2 However, increasing the active material and conductive additive concentration in the semi-solid electrode is essential to increase surface area available for electrochemical reactions.3 Similarly, one may also expect other trade-offs such as cost of electrode constituents and their electrochemical and rheological capabilities. In this study, we explore these relationships between compositions and performance, to target low-cost energy storage system.
References:
1. Duduta, M., Ho, B., Wood, V.C., Limthongkul, P., Brunini, V.E., Carter, W.C. & Chiang, Y.M. Semi-solid lithium rechargeable flow battery. Adv. Energy Mater. 1, 511–516 (2011).
2. Bonn, D., Denn, M.M., Berthier, L., Divoux, T. & Manneville, S. Yield stress materials in soft condensed matter. Rev. Mod. Phys. 89, 035005 (2017).
3. Zhu, Y., Narayanan, T.M., Tułodziecki, M., Sanchez-Casalongue, H., Horn, Q., Meda, L., Yu, Y., Sun, J., Regier, T., McKinley, G.H. & Shao-Horn, Y. High-energy and high-power Zn-Ni flow batteries with semi-solid electrodes. Sustain. Energy Fuels (2020).
The design of semi-solid electrodes for high-energy and high power flow batteries
(Credit: Yunguang Zhu)
Flow battery technology offers a promising low-cost option for stationary energy storage applications. Aqueous zinc-nickel battery chemistry is intrinsically safer than non-aqueous battery chemistry (e.g. lithium-based batteries) and offers comparable energy density.1 Unfortunately, currently-available flow batteries have energy densities (on a per volume basis) lower than Li-ion batteries.2 This is because flow battery electrodes (either catholyte or anolyte) have lower densities of redox centers per electrode volume due to limited solubility of active redox molecules (e.g. solubilities are limited to ~1 M for 9,10-anthraquinone-2,7-disulphonic acid (AQDS),3 ~2 M for VO2+ in acid4).2, 5 Therefore, it is critical to develop a new technique – the semi-solid flow battery – to boost the energy density without limitation of solubility of active materials.6 Zn-Ni semi-solid flow battery chemistry can exhibit the highest energy density (~240 Wh/Lcatholyte for 12.5 vol% of Ni(OH)2), as shown in Figure 1. Zn-Ni battery chemistry is known to have a high equilibrium voltage (~ 1.8 Vcell), which is much higher than aqueous LiTi2(PO4)3-LiFePO4 (~1.0 V)7. We are focusing on the design and optimization of conductive ZnO and Ni(OH)2 active semi-solid flowable electrodes to achieve a high-energy and high-power Zn-Ni flow battery.
We have developed a Zn-Ni semisolid flow battery (SSFB) with tunable power and energy densities.8 Structurally- and chemically-stable semi-solid electrodes were designed and demonstrated, based on a polymeric microgel (Carbopol©) dispersed in 7 M KOH electrolyte to generate a yield stress that supports electronically-conductive dense suspensions. Ex-situ X-ray diffraction and XANES analysis are used to generate the appropriate charge-discharge scenario for Zn-Ni semi-solid flow batteries. When compared with other aqueous systems, the Zn-Ni semi-solid flow battery system developed here has promising energy and power densities, which make scale-up feasible with negligible pumping energy loss. This newly-designed aqueous Zn-Ni semi-solid flow battery paves a way to develop environmentally friendly and cost-effective energy storage systems for stationary applications.
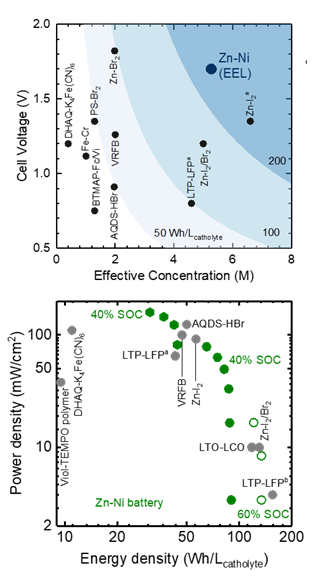
Figure 1.
References:
1. Perry, L. & Weber, A. Z. Advanced Redox-Flow Batteries: A Perspective. Journal of The Electrochemical Society, 163, A5064-A5067 (2016).
2. Huang, Q. & Wang, Q. Next‐Generation, High‐Energy‐Density Redox Flow Batteries. ChemPlusChem 80, 312-322 (2014).
3. Huskinson, B., Marshak, M. P., Suh, C., Er, S., Gerhardt, M. R., Galvin, C. J., Chen, X., Aspuru-Guzik, A., Gordon R. G. & Aziz, M. J. A metal-free organic–inorganic aqueous flow battery. Nature 505, 195 (2014).
4. Skyllas‐Kazacos, M. , Menictas C. & Kazacos, M. Thermal Stability of Concentrated V(V) Electrolytes in the Vanadium Redox Cell. Journal of the Electrochemical Society, 143, L86-L88 (1996).
5. Soloveichik, G. L. Flow Batteries: Current Status and Trends. Chemical Reviews, 115, 11533-11558 (2015).
6. Duduta, M., Ho, B., Wood, V. C., Limthongkul, P., Brunini, V. E., Carter W. C. & Chiang, Y.-M. Semi‐Solid Lithium Rechargeable Flow Battery. Advanced Energy Materials, 1, 511-516 (2011).
7. Li, Z., Smith, K. C., Dong, Y., Baram, N., Fan, F. Y., Xie, J., Limthongkul, P., Carter W. C. & Chiang, Y.-M. Aqueous semi-solid flow cell: demonstration and analysis. Physical Chemistry Chemical Physics, 15, 15833-15839 (2013).
8. Zhu, Y., Narayanan, T. M., Tułodziecki, M., Sanchez-Casalongue, H., Horn, Q., Meda, L., Yu, Y., Sun, J., Regier, T., McKinley G. H. & Shao-Horn, Y. High-energy and high-power Zn–Ni flow batteries with semi-solid electrodes. Sustainable Energy & Fuels, (2020).
Catalyst design for Li-air batteries
(Credit: Jaclyn Rose Lunger)
It has recently been shown that reversible 4-electron oxidation and reduction of lithium oxide (Li2O) is possible in a molten salt Li-O2 battery run at high temperatures and using a nickel-oxide catalyst2. While lithium peroxide (Li2O2) is the the typical discharge product in a Li-O2 battery, lithium oxide poses several significant advantages including reduced reactivity with organic solvents and an energy density rivaling that of fossil fuels. Additionally, the solubility of the lithium oxide reaction product in a molten salt electrolyte prevents the formation of a passivation layer on the cathode, improving capacity and providing an opportunity for catalyst design. However, there is currently a lack of understanding of oxygen evolution reaction (OER) kinetics in such a system and it is therefore unclear how to design catalysts for promoting 4 electron oxidation. In this work, we investigate the OER in a metal oxide based metal air battery using Density Functional Theory (DFT).
The adsorption strength of OER intermediates is calculated on an extensive library of 3d and 4d rocksalt metal oxide catalysts with DFT+U. An analogy to OER in a fuel cell is drawn, where the H+ charge carrier is replaced by Li+, Na+ and K+. While adsorbed OH* only affects the charge density of the metal atom it is adsorbed to, it is found that adsorption of OM* (where M=Li, Na, K) results in a distribution of charge. Additionally, it is found that the adsorption strength of OM* (M=H, Li, Na, or K) on a given catalyst is correlated with the atomic radii of the metal. Both insights make it possible to apply the vast literature on OER in fuel cells to catalyst design for metal air batteries. Additionally, this work highlights the possibility of catalyst design for new reactions using knowledge from previously studied reactions.
References:
1. Feng, S., Lunger, J. R., Johnson, J. A., & Shao-Horn, Y. Hot lithium-oxygen batteries charge ahead. Science, 361 (6404), 758-758 (2018).
2. Giordani, V., Tozier, D., Tan, H., Burke, C. M., Gallant, B. M., Uddin, J., Greer, J. R., McCloskey, B. D., Chase, G. V. & Addison. D. A molten salt lithium–oxygen battery. J. Am. Chem. Soc., 138 (8), 2656-2663 (2016).
3. Xia, C., Kwok, C. Y., Nazar, L. F. A high-energy-density lithium-oxygen battery based on a reversible four-electron conversion to lithium oxide. Science, 361 (6404), 777-781 (2018).