Design Principles of Metal Oxide Catalysts for Oxygen Electrocatalysis
(Credit: Jiayu Peng)
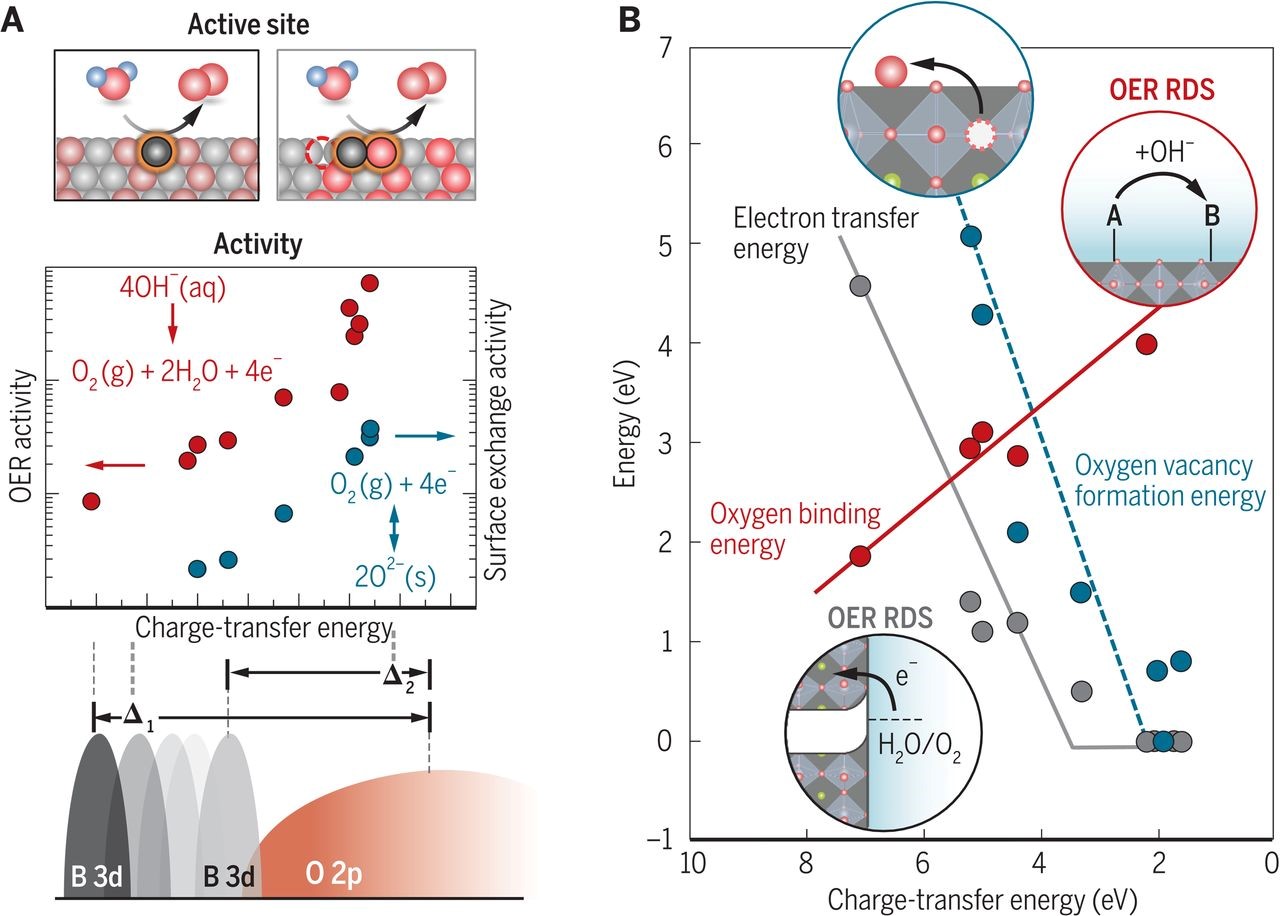
First-row transition metal oxides based on manganese, iron, cobalt and nickel have been reported to be the most active catalysts for oxygen evolution reaction (OER)1 and oxygen reduction reaction (ORR)2 in basic solution. Particularly, perovskites (ABO3-δ) characterized with immense structural, chemical and electronic flexibility associated with vast selections of A-site and B-site metal ions and oxygen deficiency3 have been studied in our group to elucidate OER/ORR mechanisms and develop activity/stability design principles for better oxide catalysts. In our earlier works, a number of electronic descriptors, such as eg antibonding orbital filling4,5 and O 2p band centers,6,7 were identified to control the OER/ORR kinetics over several orders of magnitude. Recent works have shown that lowering the charge-transfer gap or increasing metal-oxygen covalency in perovskites enhances the OER kinetics8,9 but beyond an optimal value reduces oxide stability.7 Exploiting this concept to examine a library of oxides has established a new OER mechanism for the most active oxides, where both the metal and oxygen sites can catalyze OER,8 and the chemical deprotonation from oxide surface can be rate-limiting for these oxides.9 Moreover, metal substitution with more Lewis acidic A-site ions by leveraging the inductive effect10 has been shown to regulate covalency and surface hydroxide affinity to promote the surface deprotonation and OER kinetics of highly covalent oxides.11 Applying these principles to design new oxide chemistry has led to the discovery of many active catalysts, e.g. Ba0.5Sr0.5Co0.8Fe0.2O3-δ,4 Pr0.5Ba0.5CoO3-δ7 and Bi0.2Sr0.8CoO3-δ.11
Our studies on oxide catalysts encompass extensive experimental components with model systems, e.g. the synthesis of well-defined surfaces, and the in-situ/in-operando investigation of surface and interfacial processes using advanced characterization techniques. For example, ambient pressure X-ray photoelectron spectroscopy (AP-XPS) has been utilized to identify the role of hydroxylation affinity on perovskite surfaces in ORR.12 Strong structural oscillations of Ba0.5Sr0.5Co0.8Fe0.2O3-δ particles in the presence of water vapor and electron irradiation was observed using environmental transmission electron microscopy.13 The role of surface Sr segregation and the near-surface atomic structures of LaxSr1-xCoO3-δ thin films in ORR were investigated under electrochemical operating conditions using AP-XPS and coherent Bragg rod analysis (COBRA).14 In addition, synchrotron-based surface X-ray scattering crystal truncation rod (CTR) analysis has been used to reveal the surface adsorbate structures and energetics for RuO2 single crystals with different orientations, and elucidate the influence of their local coordination environments on the OER activity.15,16
References
1. Hong, W. T., Risch, M., Stoerzinger, K. A., Grimaud, A., Suntivich, J. & Shao-Horn, Y. Toward the rational design of non-precious transition metal oxides for oxygen electrocatalysis. Energy Environ. Sci. 8, 1404–1427 (2015).
2. Stoerzinger, K. A., Risch, M., Han, B. & Shao-Horn, Y. Recent Insights into Manganese Oxides in Catalyzing Oxygen Reduction Kinetics. ACS Catal. 5, 6021–6031 (2015).
3. Hwang, J., Rao, R. R., Giordano, L., Katayama, Y., Yu, Y. & Shao-Horn, Y. Perovskites in catalysis and electrocatalysis. Science 358, 751–756 (2017).
4. Suntivich, J., May, K. J., Gasteiger, H. A., Goodenough, J. B. & Shao-Horn, Y. A perovskite oxide optimized for molecular orbital principles. Science 334, 1383–1385 (2011).
5. Suntivich, J., Gasteiger, H. A., Yabuuchi, N., Nakanishi, H., Goodenough, J. B. & Shao-Horn, Y. Design principles for oxygen-reduction activity on perovskite oxide catalysts for fuel cells and metal-air batteries. Nat. Chem. 3, 546–550 (2011).
6. Lee, Y. L., Kleis, J., Rossmeisl, J., Shao-Horn, Y. & Morgan, D. Prediction of solid oxide fuel cell cathode activity with first-principles descriptors. Energy Environ. Sci. 4, 3966–3970 (2011).
7. Grimaud, A., May, K. J., Carlton, C. E., Lee, Y.-L., Risch, M., Hong, W. T., Zhou, J. & Shao-Horn, Y. Double perovskites as a family of highly active catalysts for oxygen evolution in alkaline solution. Nat. Commun. 4, 2439 (2013).
8. Grimaud, A., Diaz-Morales, O., Han, B., Hong, W. T., Lee, Y. L., Giordano, L., Stoerzinger, K. A., Koper, M. T. M. & Shao-Horn, Y. Activating lattice oxygen redox reactions in metal oxides to catalyse oxygen evolution. Nat. Chem. 9, 457–465 (2017).
9. Hong, W. T., Stoerzinger, K. A., Lee, Y.-L., Giordano, L., Grimaud, A. J. L., Johnson, A. M., Hwang, J., Crumlin, E. J., Yang, W. & Shao-Horn, Y. Charge-transfer-energy-dependent oxygen evolution reaction mechanisms for perovskite oxides. Energy Environ. Sci. 10, 2190–2200 (2017).
10. Kuznetsov, D. A., Han, B., Yu, Y., Rao, R. R., Hwang, J., Román-Leshkov, Y. & Shao-Horn, Y. Tuning redox transitions via inductive effect in metal oxides and complexes, and implications in oxygen electrocatalysis. Joule 2, 225–244 (2018).
11. Kuznetsov, D. A., Peng, J., Giordano, L., Román-Leshkov, Y. & Shao-Horn, Y. Bismuth Substituted Strontium Cobalt Perovskites for Catalyzing Oxygen Evolution. J. Phys. Chem. C 124, 6562–6570 (2020).
12. Stoerzinger, K. A., Hong, W. T., Wang, X. R., Rao, R. R., Bengaluru Subramanyam, S., Li, C., Ariando, Venkatesan, T., Liu, Q., Crumlin, E. J., Varanasi, K. K. & Shao-Horn, Y. Decreasing the Hydroxylation Affinity of La1–xSrxMnO3 Perovskites To Promote Oxygen Reduction Electrocatalysis. Chem. Mater. 29, 9990–9997 (2017).
13. Han, B., Stoerzinger, K. A., Tileli, V., Gamalski, A. D., Stach, E. A. & Shao-Horn, Y. Nanoscale structural oscillations in perovskite oxides induced by oxygen evolution. Nat. Mater. 16, 121–126 (2016).
14. Feng, Z., Hong, W. T., Fong, D. D., Lee, Y.-L., Yacoby, Y., Morgan, D. & Shao-Horn, Y. Catalytic Activity and Stability of Oxides: The Role of Near-Surface Atomic Structures and Compositions. Acc. Chem. Res. 49, 966–973 (2016).
15. Rao, R. R., Kolb, M. J., Halck, N. B., Pedersen, A. F., Mehta, A., You, H., Stoerzinger, K. A., Feng, Z., Hansen, H. A., Zhou, H., Giordano, L., Rossmeisl, J., Vegge, T., Chorkendorff, I., Stephens, I. E. L. & Shao-Horn, Y. Towards identifying the active sites on RuO2(110) in catalyzing oxygen evolution. Energy Environ. Sci. 10, 2626–2637 (2017).
16. Rao, R. R., Kolb, M. J., Giordano, L., Pedersen, A. F., Katayama, Y., Hwang, J., Mehta, A., You, H., Lunger, J. R., Zhou, H., Halck, N. B., Vegge, T., Chorkendorff, I., Stephens, I. E. L. & Shao-Horn, Y. Operando identification of site-dependent water oxidation activity on ruthenium dioxide single-crystal surfaces. Nat. Catal. (2020).
Design of active catalytic environments through electrolyte engineering
(Credit: Botao Huang)
Departing from the traditional descriptor based on the surface electronic structure for electron transfer kinetics, tuning non-covalent interactions at the electrified interfaces through electrolyte engineering is an alternative pathway to design energy-conversion and energy-storage devices. A growing list of examples show that the catalytic activity1–3 and selectivity4,5 of electron transfer processes can be governed by the physical chemistry of electrolytes. For example, the exchange current density of HER/HOR (hydrogen evolution/oxidation reactions) on platinum-group metals in acidic electrolytes is ~2 orders of magnitude higher than that in alkaline solutions and decreases linearly with increasing pH.6 Moreover, the electrocatalytic activity of HOR, ORR (oxygen reduction reaction) and methanol oxidation on platinum catalyst at 0.9 VRHE was shown to increase in the order of Li+ < Na+ < K+ < Cs+.2 Similar trends have also been noted for OER (oxygen evolution reaction) kinetics on iridium oxide (IrO2)7,8 and nickel oxyhydroxide (NiOOH). In addition to altering activity, spectator cations have also shown to modulate the selectivity.9 In aqueous CO2 reduction, the cathodic activity increases from Li+ to Cs+ over Ag and Cu surfaces with a decrease in Faradaic efficiencies for H2 and CH4, and an increase in Faradaic efficiencies for CO, C2H4 and C2H5OH. In aqueous CO reduction, Rb+ and Cs+ was shown to enhanced selectivity toward C2H4, while Li+, Na+ and K+ instead promote the CH4 pathway4. All these observations point to the chemical physics of electrolytes significantly affecting reaction kinetics and selectivity. The question then becomes how pH and cation alter the structure and dynamics of the electrode/electrolyte interface and modify the energetics of reaction intermediates. It is still a great challenge to provide a molecular-level understanding of the contribution of non-covalent interactions associated with spectator ions, water molecules, redox active species and electrode surface to the electron transfer processes either in the redox solvation shell or at electrified interfaces.
In addition to surface electronic structure descriptors, we propose an alternative path that leverages physical chemistry of liquids, namely the water structure at outer-Helmholtz layer (OHL), to control the electrochemical reaction kinetics. This part of project proposes to explore the influence of water structure though electrolyte engineering on both outer-sphere and inner-sphere electron transfer (ET) processes for energy conversion and storage reactions. Combining the experimental results in both conventional and concentrated electrolytes, and energetics/dynamics from computation, we aim to establish the correlation between electron transfer kinetic parameters and interfacial layer structure during (proton coupled) electron transfer processes from electrolyte engineering, which could gain insights for the design of high-efficient energy conversion and storage devices.
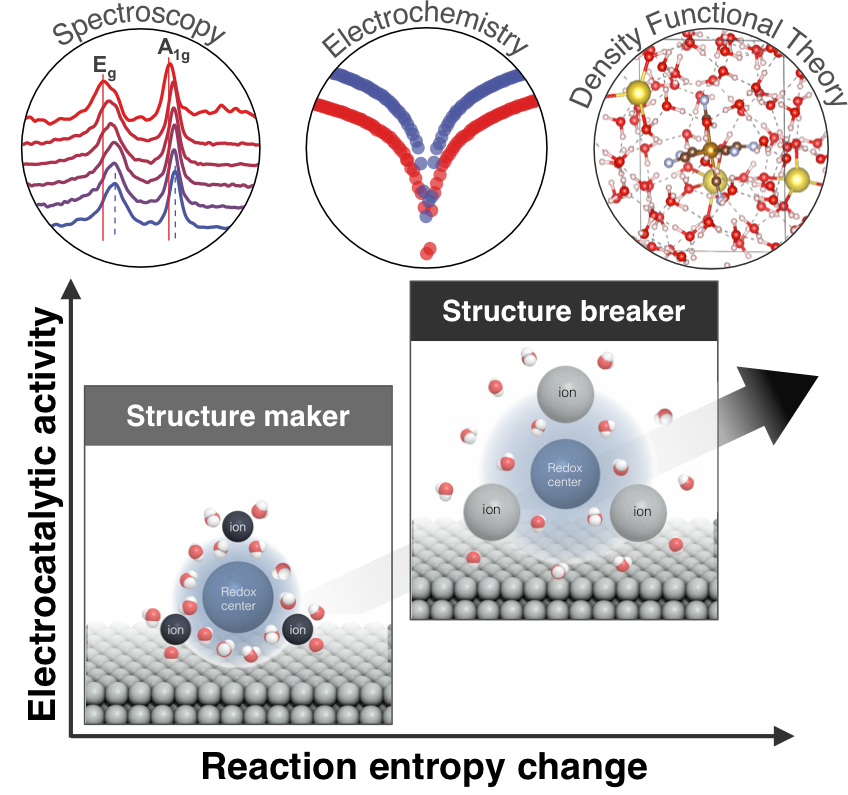
Figure 1. Tuning redox solvation shell disordering was suggested to control reaction entropy change and redox kinetics in thermal electrochemical conversion.
References
1. Zheng, J., Sheng, W., Zhuang, Z., Xu, B. & Yan, Y. Universal dependence of hydrogen oxidation and evolution reaction activity of platinum-group metals on pH and hydrogen binding energy. Sci Adv 2, (2016).
2. Strmcnik, D. et al. The role of non-covalent interactions in electrocatalytic fuel-cell reactions on platinum. Nat Chem 1, 466–472 (2009).
3. Ledezma-Yanez, I. et al. Interfacial water reorganization as a pH-dependent descriptor of the hydrogen evolution rate on platinum electrodes. Nature Energy 2, 17031 (2017).
4. Pérez-Gallent, E., Marcandalli, G., Figueiredo, M. C., Calle-Vallejo, F. & Koper, M. T. M. Structure- and Potential-Dependent Cation Effects on CO Reduction at Copper Single-Crystal Electrodes. J. Am. Chem. Soc. 139, 16412–16419 (2017).
5. Singh, M. R., Kwon, Y., Lum, Y., Ager, J. W. & Bell, A. T. Hydrolysis of Electrolyte Cations Enhances the Electrochemical Reduction of CO2 over Ag and Cu. J. Am. Chem. Soc. 138, 13006–13012 (2016).
6. Durst, J. et al. New insights into the electrochemical hydrogen oxidation and evolution reaction mechanism. Energy Environ. Sci. 7, 2255–2260 (2014).
7. Suntivich, J., Perry, E. E., Gasteiger, H. A. & Shao-Horn, Y. The Influence of the Cation on the Oxygen Reduction and Evolution Activities of Oxide Surfaces in Alkaline Electrolyte. Electrocatalysis 4, 49–55 (2013).
8. Kuo, D.-Y. et al. Influence of Surface Adsorption on the Oxygen Evolution Reaction on IrO2(110). J. Am. Chem. Soc. 139, 3473–3479 (2017).
9. Garcia, A. C., Touzalin, T., Nieuwland, C., Perini, N. & Koper, M. T. M. Enhancement of Oxygen Evolution Activity of Nickel Oxyhydroxide by Electrolyte Alkali Cations. Angewandte Chemie International Edition 58, 12999–13003 (2019).
10. Sheng, W. et al. Correlating hydrogen oxidation and evolution activity on platinum at different pH with measured hydrogen binding energy. Nature Communications 6, 5848 (2015).
11. Rossmeisl, J., Chan, K., Skúlason, E., Björketun, M. E. & Tripkovic, V. On the pH dependence of electrochemical proton transfer barriers. Catalysis Today 262, 36–40 (2016).
Beyond metal oxides: metal-organic framework (MOF)-based electrocatalysts with enhanced tunability
(Credit: Shuai Yuan)
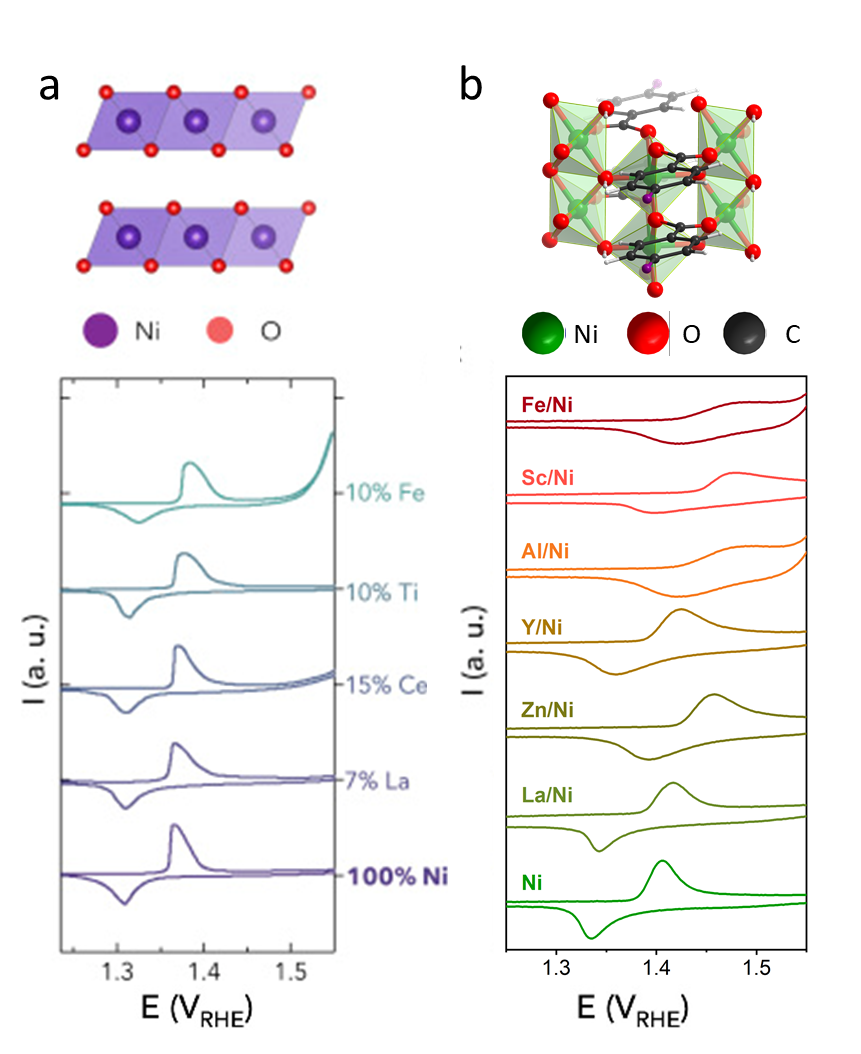
Beyond metal oxides, we have employed MOFs as platforms to design electrocatalysts that combine the tunability of molecular catalysts with the practical advantages of heterogeneous catalysts. MOFs1 are an emerging class of hybrid materials constructed from metal clusters and organic ligands. Compared with traditional heterogenous electrocatalysts such as metal oxides, MOFs possess unprecedented tunability by modifying metal components and organic ligands. We have demonstrated that the electronic structure of Ni-oxide clusters in MOFs can be fine-tuned by the inductive effect through partial metal substitution.2 The potential of the Ni redox peak (Figure 1b) was found to shift considerably to positive potentials with more acidic cations (i.e. electron-withdrawing capability) in basic solutions, where the shift associated with MOFs (~0.1 V) was five times larger than that measured for substituted nickel hydroxides (~0.02 V in Figure 1a), indicating that the electronic structures of MOFs is more tunable than traditional metal-oxide based catalysts. By tuning the electronic structures, the oxygen evolution reaction (OER) activity of Ni-MOFs was enhanced by three orders of magnitude, leading to a optimized electrocatalyst with higher mass activity than benchmark IrO2 under alkaline conditions. Future works will focus on the design of tunable MOF-based electrocatalysis for a variety of electrocatalysis including OER, oxygen reduction reaction, and CO2 reduction reaction.
References
1. Yuan, S., Peng, J., Zhang, Y., Shao-Horn, Y. Stability Trend of Metal–Organic Frameworks with Heterometal-Modified Hexanuclear Zr Building Units. J. Phys. Chem. C 123, 28266-28274 (2019).
2. Kuznetsov, D. A., Han, B., Yu, Y., Rao, R. R., Hwang, J., Román-Leshkov, Y., Shao-Horn, Y. Tuning Redox Transitions Via Inductive Effect in Metal Oxides and Complexes, and Implications in Oxygen Electrocatalysis. Joule 2, 225-244 (2018).
3. Enman, L. J., Burke, M. S., Batchellor, A. S., Boettcher, S. W. Effects of Intentionally Incorporated Metal Cations on the Oxygen Evolution Electrocatalytic Activity of Nickel (Oxy)Hydroxide in Alkaline Media. ACS Catal. 6, 2416-2423 (2016).
Heterogeneous Catalysis for a Sustainable Economy
(Credit: Karthik Akkiraju)
Industrial heterogeneous catalysts account for 35-40% of global Gross Domestic Product (GDP) of the world and are involved in 95% of total volume of the products of the chemical industry thereby forging a close link between economic growth and sustainability. With an ever-growing economy comes a need to maintain a sustainable chemistry industry supply pathway and historically heterogeneous catalysts have altered the course of human development. For example, the humans supported on an arable land increased from 1.9 to 4.3 persons with increase of 30-50% crop yield due to the Haber-Bosch process4, silver catalysts increased selective epoxidation of ethylene to from 50% to 90% 5 in the chemical industry while three-way catalysts reduced emissions in gasoline engines by more than 98%.
With the energy crisis and global warming fast dawning upon, there is a need to improve the existing infrastructure as well as investigate alternative strategies for a sustainable future. Natural gas has been consistently mentioned as the fuel of the future alongside renewables to replace crude oil and fossil fuels. 4,5. Large fraction (75 – 90%) of natural gas is methane followed by smaller amounts of propane and butane. 6-8Methane is a greenhouse gas 25 times more impactful than CO2 and the flaring of methane (3.5% in 2012) remains an issue that limits the widespread adoption of natural gas. Addressing selective methane oxidation provides a twofold solution to tackle the global warming issues as well as the energy needs. As methane transport is not economically viable, strategies are being devised convert methane into value-added fuels such as C2 compounds (oxidative coupling of methane, OCM) 11, benzene (methane dehydroaromatization, MDA) 12, methanol11, formaldehyde12, and also propane into propene 13 (ODH) for the chemical industry with the goal of local production and easy transportation. Selective oxidation of methane (and higher alkanes) to any of the value added chemicals has been a long standing challenge with the limitation of low selectivity at high conversion due to the inert nature of the C-H bond and the ease of activation of intermediates that are formed after the initial C-H activation. Selectivity and conversion tradeoff is a well-known limitation for selective oxidation applications such as methane to methanol14 conversion and also propane conversion to propene15 and not many catalysts feature above the industrial requirement. Although, various strategies have been proposed to overcome the selectivity-conversion limitations including the use of aqueous reaction conditions, tuning the reaction conditions, usage of methanol collectors11, and bio-inspired catalyst designs16, the development of new materials with better yields remains a holy grail in chemistry.
The challenge of selective oxidation is not only limited to alkane oxidation reactions but is a common theme encountered in heterogeneous catalytic reactions such as hydrogen peroxide production during ORR17, production of value added fuels during CO2 reduction18, selective oxidation of alcohols and aldehydes19. Selective oxidation reactions present a challenge due to the difficulty in controlling subsequent dehydrogenation after the high activation energy of first bond dissociation energy. While efficient catalysts for selective oxidation remain to be researched, the importance of improving activity and stability of catalysts for reactions such as oxygen evolution reaction, oxygen reduction reaction, hydrogen evolutions, nitrogen reduction in the context of meeting energy needs cannot be understated. In this work, we aim to develop a framework to address this challenge by systematically tuning the electronic structure of the catalysts as well as the environmental conditions using both experimental and computational approaches.
References
1. Chu, S. & Majumdar, A. Opportunities and challenges for a sustainable energy future. Nature 488, 294–303 (2012).
2. Deutschmann, O., Knözinger, H., Kochloefl, K. & Turek, T. Heterogeneous Catalysis and Solid Catalysts, 3. Industrial Applications. in Ullmann’s Encyclopedia of Industrial Chemistry (Wiley-VCH Verlag GmbH & Co. KGaA, 2011).
3. Lanzafame, P., Perathoner, S., Centi, G., Gross, S. & Hensen, E. J. M. Grand challenges for catalysis in the Science and Technology Roadmap on Catalysis for Europe: moving ahead for a sustainable future. Catal. Sci. Technol. 7, 5182–5194 (2017).
4. Statistical Review of World Energy | Energy economics | BP. (Accessed: 30th November 2018)
5. The Future of Natural Gas: An Interdisciplinary MIT Study.
6. Grant, J. T., Venegas, J. M., McDermott, W. P. & Hermans, I. Aerobic Oxidations of Light Alkanes over Solid Metal Oxide Catalysts. Chem. Rev. 118, 2769–2815 (2018).
7. Tollefson, J. ‘Flaring’ wastes 3.5% of world’s natural gas. Nature (2016).
8. Schlgl, R. Concepts in Selective Oxidation of Small Alkane Molecules. in Modern Heterogeneous Oxidation Catalysis 1–42 (Wiley-VCH Verlag GmbH & Co. KGaA).
9. Olah, G. A. Beyond Oil and Gas: The Methanol Economy. Angew. Chemie Int. Ed. 44, 2636–2639 (2005).
10. Liu, W.-C., Baek, J. & Somorjai, G. A. The Methanol Economy: Methane and Carbon Dioxide Conversion. Top. Catal. 61, 530–541 (2018).
11. Latimer, A. A., Kakekhani, A., Kulkarni, A. R. & Nørskov, J. K. Direct Methane to Methanol: The Selectivity−Conversion Limit and Design Strategies. (2018).
12. Carrero, C. A., Schloegl, R., Wachs, I. E. & Schomaecker, R. Critical Literature Review of the Kinetics for the Oxidative Dehydrogenation of Propane over Well-Defined Supported Vanadium Oxide Catalysts. ACS Catal. 4, 3357–3380 (2014).
13. Schwach, P., Pan, X. & Bao, X. Direct Conversion of Methane to Value-Added Chemicals over Heterogeneous Catalysts: Challenges and Prospects. Chem. Rev. 117, 8497–8520 (2017).
14. Ravi, M., Ranocchiari, M. & van Bokhoven, J. A. The Direct Catalytic Oxidation of Methane to Methanol-A Critical Assessment. Angew. Chemie Int. Ed. 56, 16464–16483 (2017).
15. Cavani, F. & Trifirò, F. The oxidative dehydrogenation of ethane and propane as an alternative way for the production of light olefins. Catal. Today 24, 307–313 (1995).
16. Labinger, J. A. & Bercaw, J. E. Understanding and exploiting C–H bond activation. Nature 417, 507–514 (2002).
17. Yang, S. et al. Toward the Decentralized Electrochemical Production of H2O2 : A Focus on the Catalysis. ACS Catal. 8, 4064–4081 (2018).
18. Zhu, D. D., Liu, J. L. & Qiao, S. Z. Recent Advances in Inorganic Heterogeneous Electrocatalysts for Reduction of Carbon Dioxide. Adv. Mater. 28, 3423–3452 (2016).
19. Putin, E. et al. Reinforced Adversarial Neural Computer for de Novo Molecular Design. J. Chem. Inf. Model. 58, 1194–1204 (2018).