Research
Our group is interested in developing next-generation optical imaging and computational methods that will enable noninvasive (label-free), deeper, faster, and richer (more contrast) visualization of biosystems.
Current research:
Label-free Nonlinear Microscopy
Ultrabroadband Light Source
AI-assisted Microscopy
High-Speed Wavefront Shaping
Label-free Nonlinear Microscopy
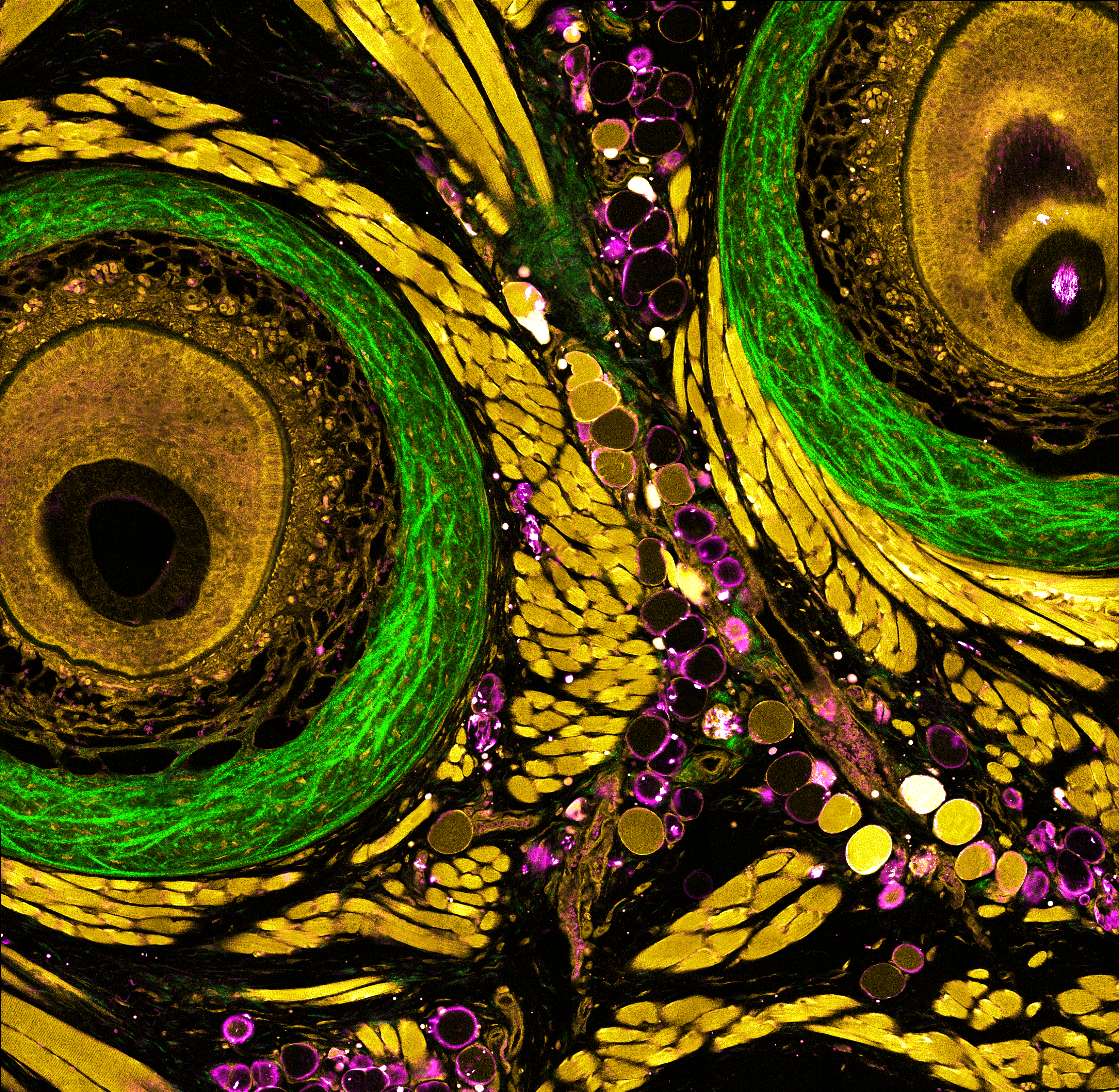
Understanding of complex diseases, therapeutic effects, and biological functions requires new imaging tools with minimal biological disruption, multiplexed molecular contrast in high volumes and high resolution. We are developing next-generation microscopy methods that will enable noninvasive (label-free), deeper, faster, and richer (more contrast) visualization of biosystems. The example images are acquired in collaboration with Wang Lab at BCS.
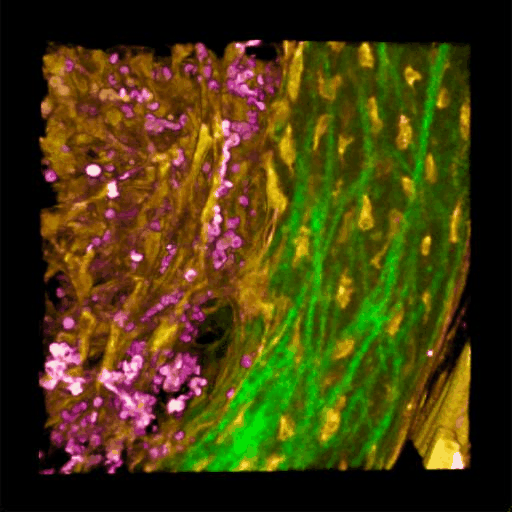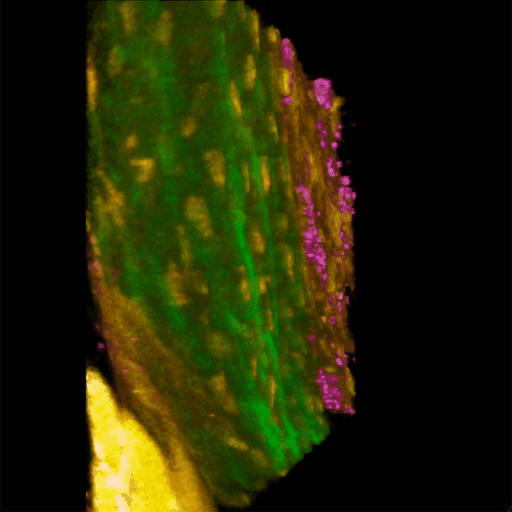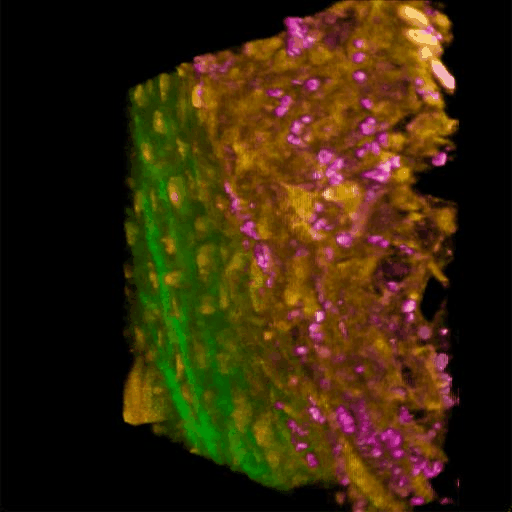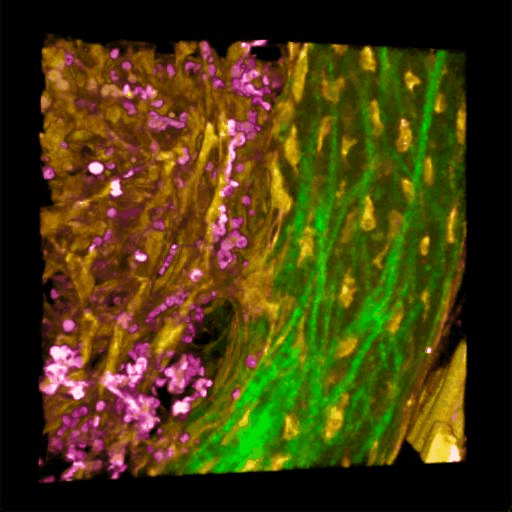
Ultrabroadband Light Source
Advances in nonlinear optical imaging have been accelerated with advances in ultrafast light sources. With the increasing demand of multiplexing, imaging depth, and speed, there is an urgent need for more versatile and flexible light sources. By leveraging nonlinear fiber optics and feedback-based control, our lab is exploring new ways of generating tunable broadband fiber sources for advanced bioimaging.
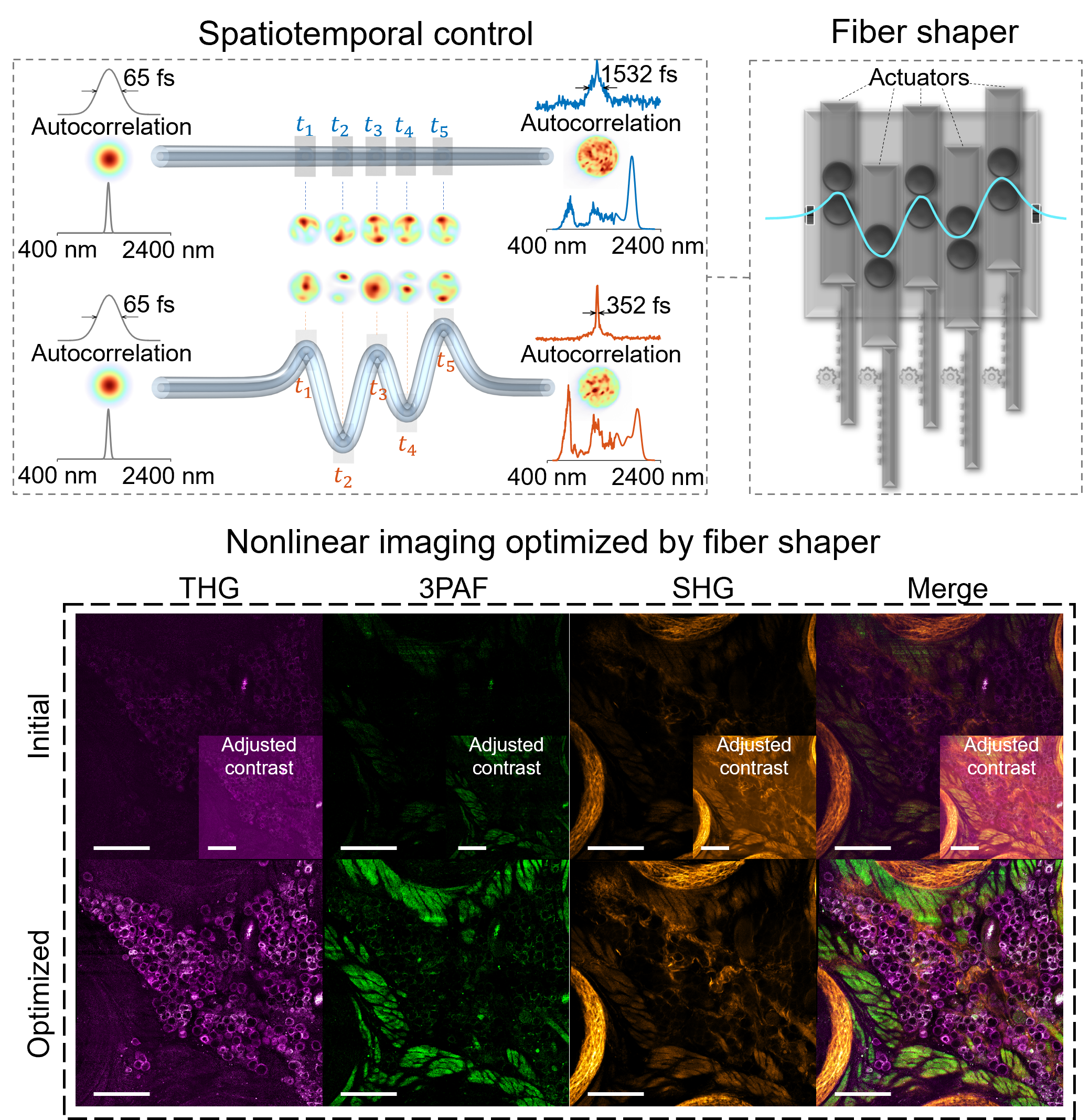
AI-assisted Microscopy
Beyond the conventional optical methods, the integration of AI algorithms also plays an important role in the ongoing quest for label-free visualization of biosystems. Physics-based AI could address physically limited challenges like isotropic resolution, low-light imaging, and lack of molecular specificity. It enables us to explore the intricacies of life at the microscopic level with unprecedented clarity and depth, thereby opening doors to new discoveries and innovative applications across diverse fields.

High-Speed Wavefront Shaping
Engineering the wavefront enables light shaping through complex media, crucial in holography, optical communications, and bioimaging. However, balancing speed and accuracy is challenging due to hardware limits, causing fast projections to lack fidelity. This can be mitigated by integrating hardware, physics, and algorithms, designing optimizations aligned with the physical constraints of light modulation devices.
We leverage complex media to convert high-bandwidth signals in the visualization domain to low-bandwidth signals in the control domain, overcoming hardware bandwidth limits and enhancing holography fidelity at high speeds. This promises advancements in applications like computer-generated holography, multimode-fiber-based communications, and deep tissue imaging.
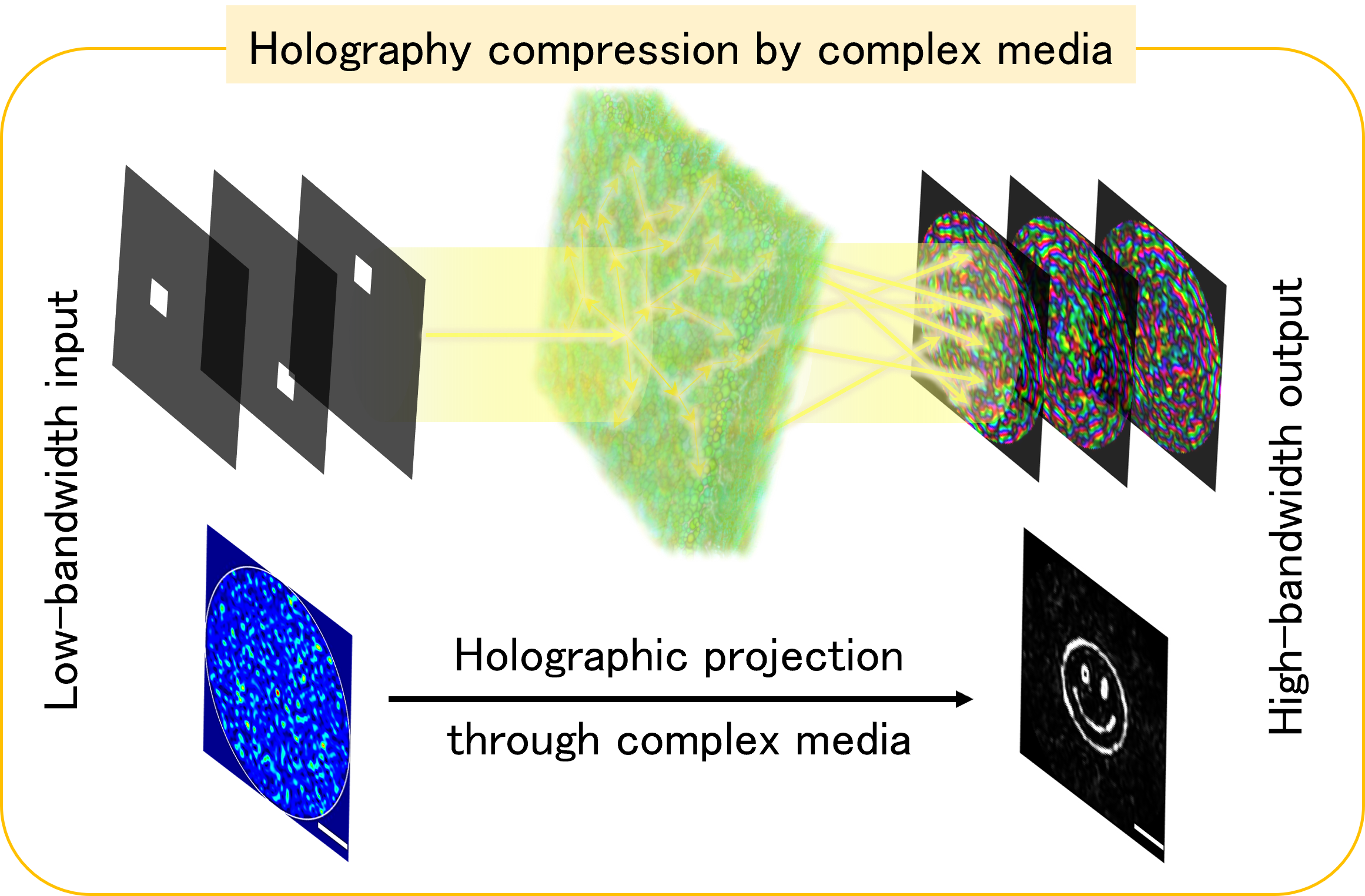
Previous research:
Development of Label-free multiphoton microscopy
Label-free in vivo microscopy
AI-driven translation into clinic
Raman spectroscopy and imaging
Label-free multiphoton microscopy
Using fiber supercontinuum and pulse shaping, we developed a multiphoton imaging platform that achieves real-time label-free single-shot functional and structural optical imaging for the first time, and enables non-perturbative, faster, and richer visualization of living systems.
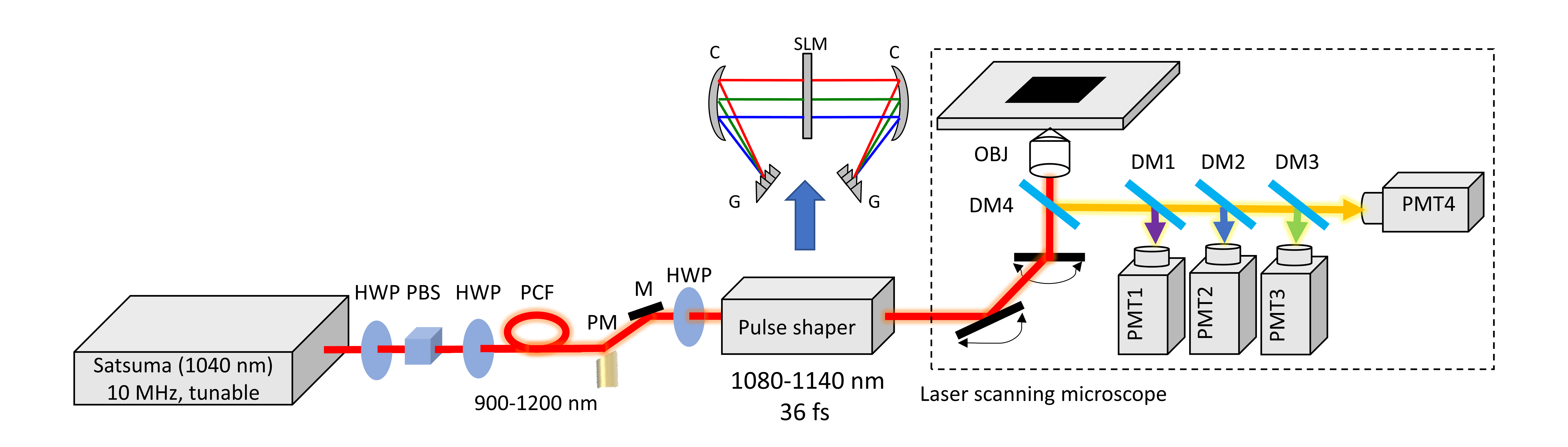
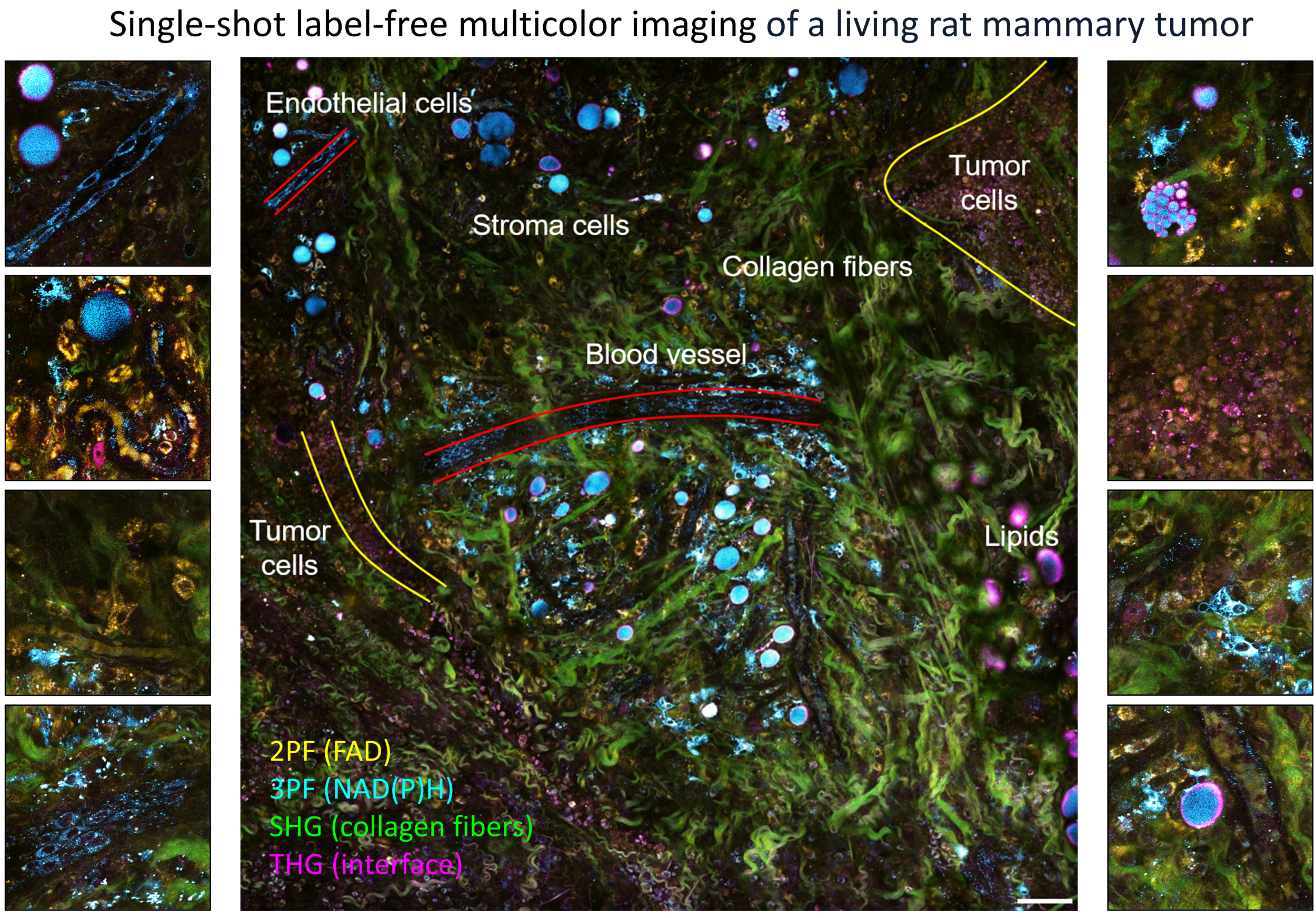
Lable-free in vivo microscopy
Because of the nonlinear dependence on incident power and the longer excitation wavelength, multiphoton imaging achieves deeper imaging with less photobleaching artifacts compared to one-photon imaging. This opens windows to in vivo imaging of the dynamic living biosystems. Label-free multiphoton microscopy makes the technique even more attractive by the efficient excitation and detection of the intrinsic molecular contrasts.
By utilizing the intrinsic metabolic signatures of biological molecules, we were able to directly visualize and characterize extracellular vesicles in living animals and fresh human tissues with breast cancer.
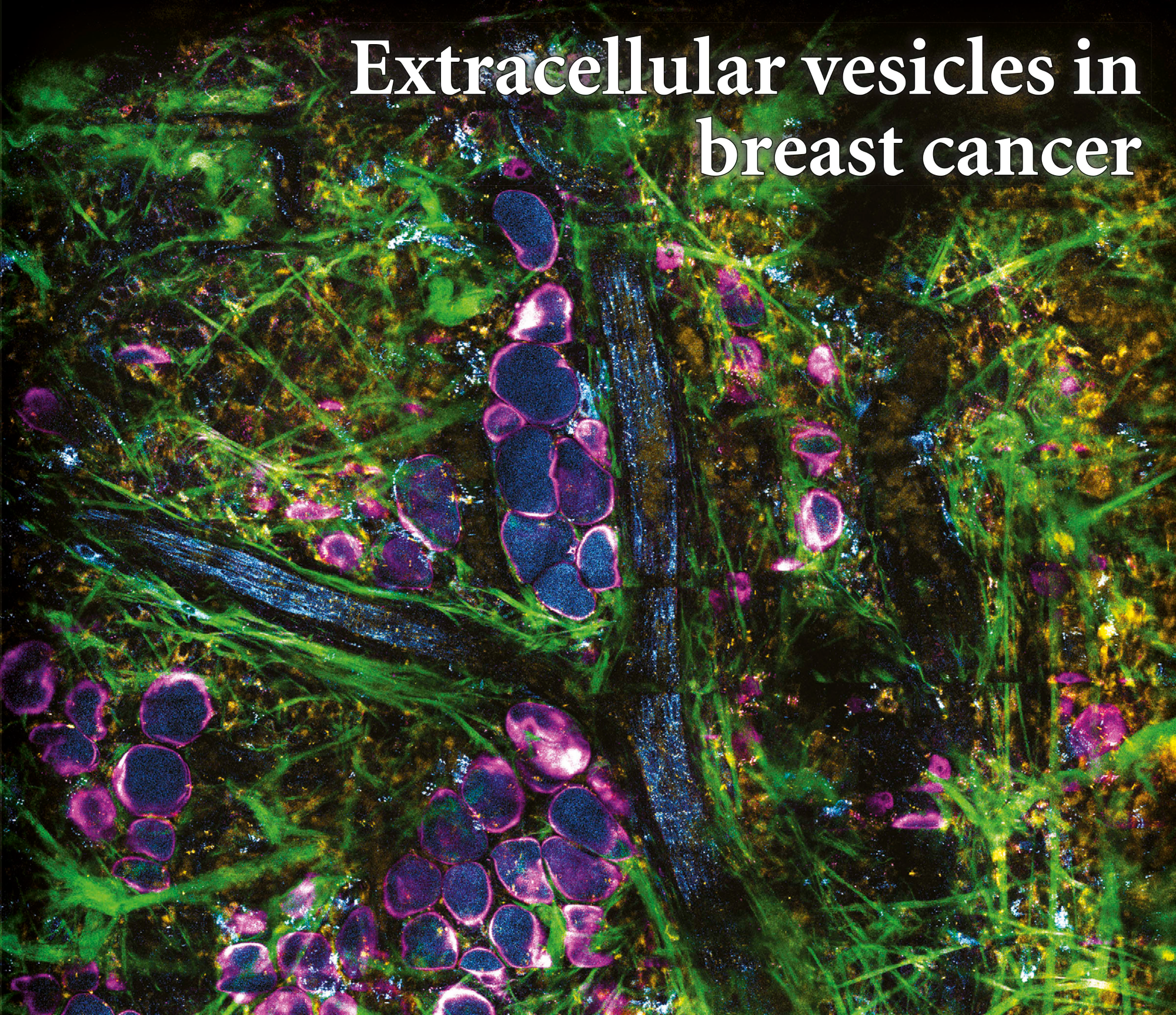
AI-driven translation into clinic
The high (~30%) re-operation rate for breast conserving surgeries arise from the failure of detecting positive margins at the time of surgery. This risk can be significantly reduced by a combined framework of real-time virtual histopathology and a deep-learning-based classification algorithm.
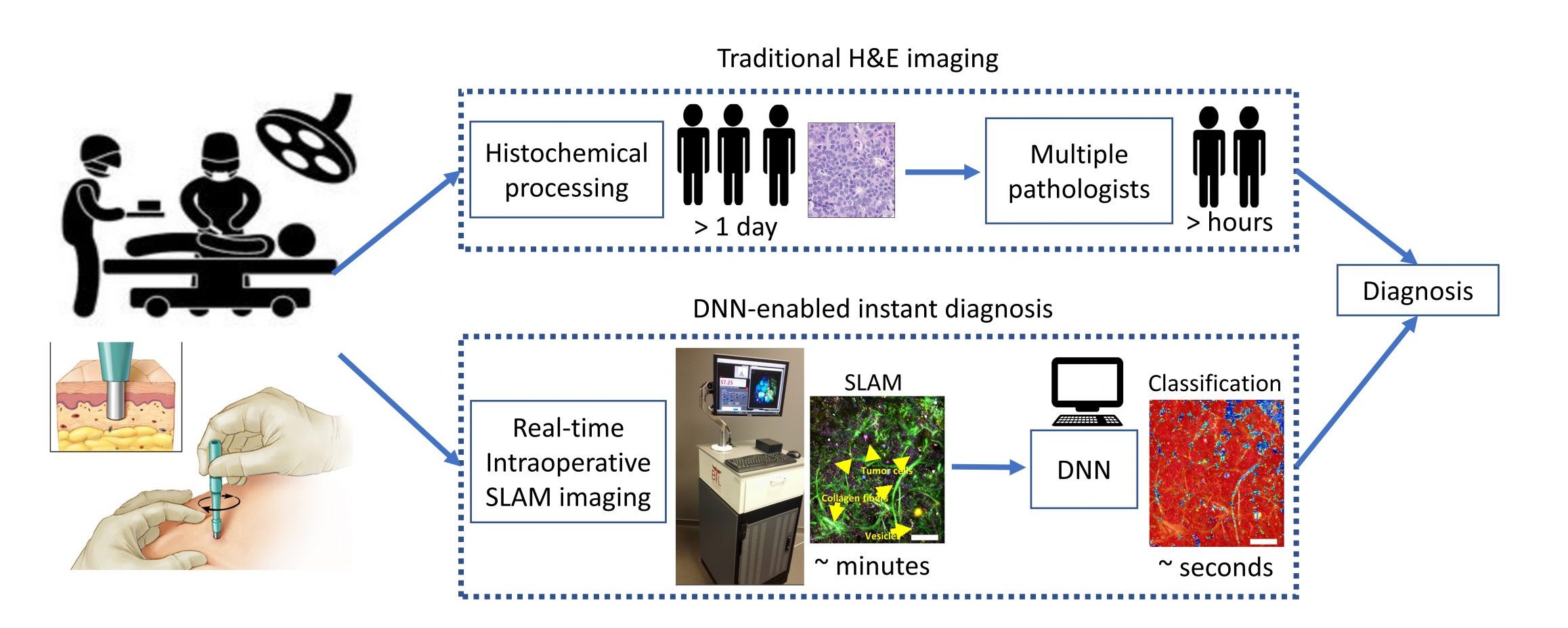
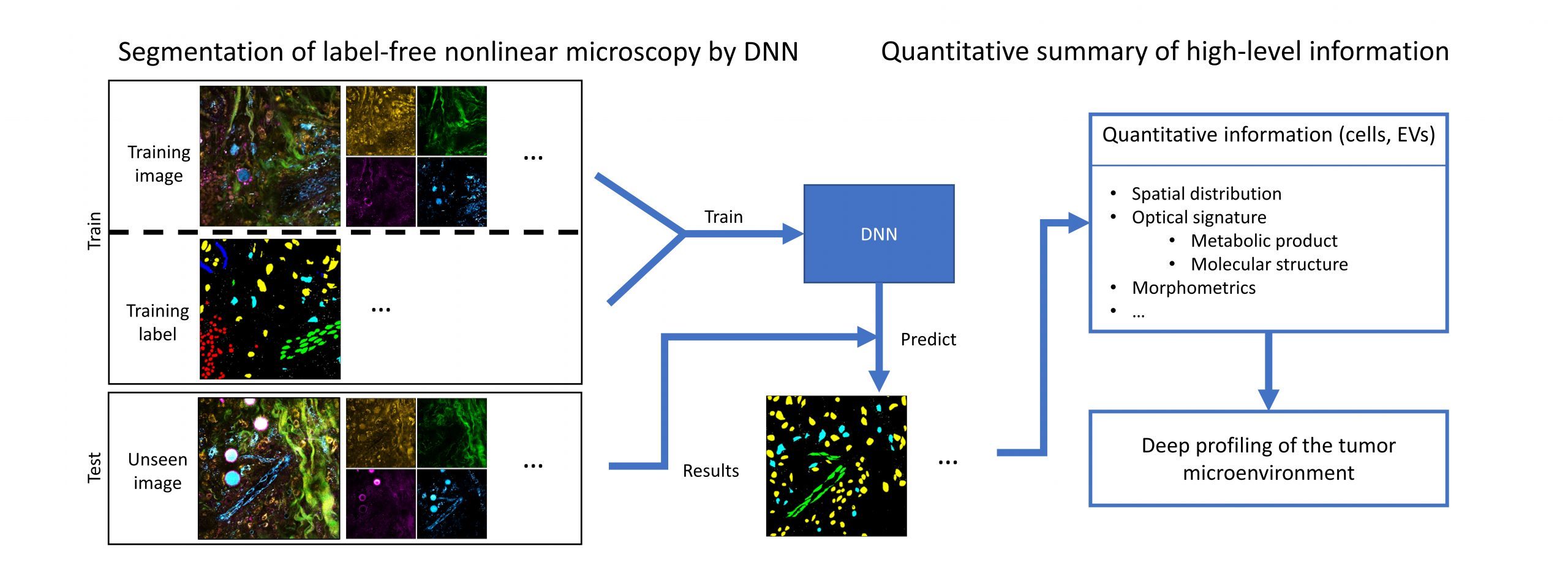
Recent advances in microscopy, along with advances in artificial intelligence (AI), present an exciting opportunity to innovate microscopy analysis and instrumentation. Here is an example of how we use deep learning to “pseudo label” the label-free images.
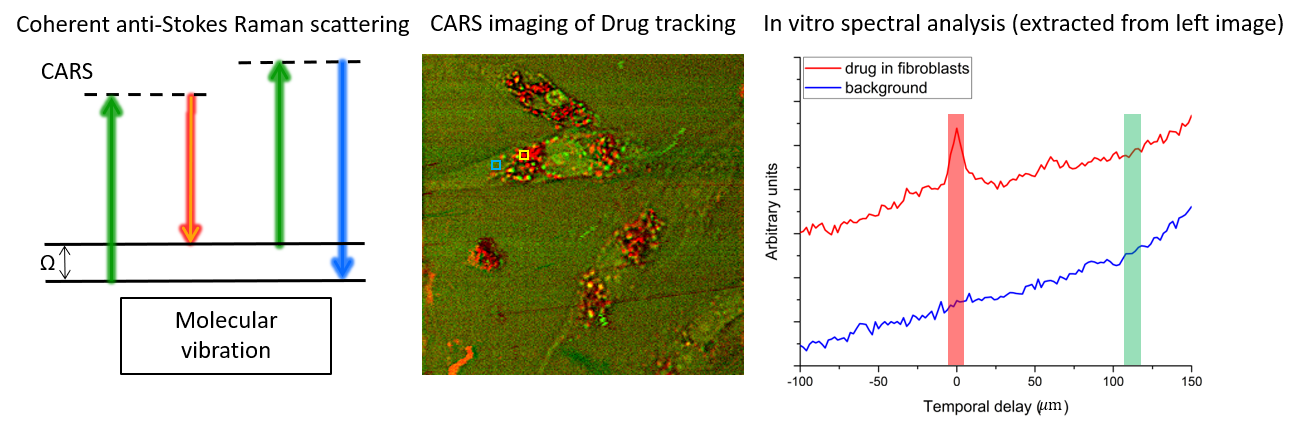
Raman spectroscopy and coherent Raman imaging
Raman spectroscopy provides noninvasive fingerprints of chemical compositions at molecular level. Coherent Raman scattering makes it possible to extend this chemical profiling capacity to broader imaging applications that require high spatiotemporal resolution on top of the chemical resolution, e.g. drug tracking in living cells as shown in the figure below. Signal throughput has been a longstanding limit to further expand this technique to real-life biomedical applications. We aim to develop methods that can address this issue by efficiently acquiring and reconstructing the hyperspectral dataset.