M. Tuodziecki, G. M. Leverick, C. V. Amanchukwu, Y. Katayama, D. G. Kwabi, F. Bardé, P. T. Hammond and Y. Shao-Horn
DOI: 10.1039/C7EE00954B
Abstract:
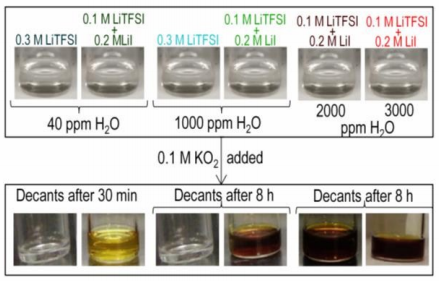
Lithium iodide has been studied extensively as a redox-mediator to reduce the charging overpotential of Li-oxygen (Li-O2) batteries. Ambiguities exist regarding the influence of lithium iodide on the reaction product chemistry and performance of lithium-oxygen batteries. In this work, we examined the role of lithium iodide on the reduction product chemistry under two conditions: i) mixing KO2 with lithium salts and ii) discharging Li-oxygen batteries at high and low overpotentials, in the presence of an ether-based electrolyte with different ratios of H2O:LiI. The addition of iodide to electrolytes containing water was found to promote the formation of LiOOH·H2O, LiOH·H2O and LiOH at the expense of Li2O2. At low H2O:LiI ratios (lower than 5), LiOH instead of Li2O2 was formed, which was accompanied by the oxidation of iodide to triodide while at high H2O:LiI ratios (12, 24, 134), a mixture of Li2O2, LiOOH·H2O and LiOH·H2O was observed and no triiodide was detected. The reaction between peroxide Li2O2 and/or superoxide LiO2 with H2O to form LiOH is facilitated by increased water acidity by strong I- ‑H2O interactions as revealed by 1HNMR and FT-IR measurements. This mechanism of LiOH formation in the presence of LiI and H2O was also found upon Li-O2 cell discharge, which is critical to consider when developing LiI as a redox mediator for Li-O2 batteries.
