Understanding Transport in Single Ion Conducting Polymers
(Credit: Michael Stolberg)
Polymer electrolytes are a safe alternative to conventional non-aqueous electrolytes used in modern day lithium ion batteries. Although they have been studied since the early 1980’s, polymer electrolytes have not realized wide spread adoption due to their sub-par ion transport properties.1 Polymer electrolytes suffer from low ionic conductivity and low transference numbers, typically less than 0.5, indicating that the majority of the ionic current is due to transport of the anion, which is deleterious to battery operation.2 We seek to further understand ion transport through polymeric media, and leverage this insight to produce higher performing and safe polymeric electrolytes.
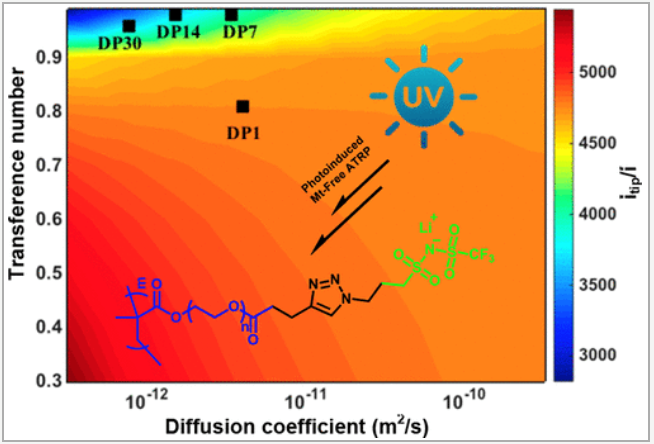
One class of materials the EEL lab is interested in are single ion conducting polymers, wherein the anion is covalently bound to the polymer. Immobilizing the anion results in near transference numbers approaching 1 due to the slow diffusion of the polymeric anion3,4. High transference numbers have been shown to impart beneficial properties the battery. In addition to making all the ionic conductivity productive towards the redox reactions, they have also been shown to prevent the formation of concentration gradients through the electrolyte.5 Furthermore, they have also been shown to limit dendrite growth when used with high energy density lithium metal electrodes.4 Figure 1 exemplifies this, where computational models show that having low transference numbers result in higher currents at the tip of dendrites, thought to accelerate dendrite growth. Multiple ion pairs have been used for single ion polymers, and it is generally found that the lower the dissociation energy of the ionic group, the higher the ionic conductivity.6 However even with highly dissociative anions SICPs struggle to attain ionic conductivities >10-4 S/cm at elevated temperatures.
To create high performance single ion conducting polymers we work closely with the Johnson group at MIT. Together we utilize advanced polymer synthesis techniques to incorporate novel and tunable anions into polymers of various architectures. Synthetic modifications can be made to alter the electronic structures of the polymers, altering the dissociation energy. We then use electrochemical methods to characterize the ion transport properties. X-ray spectroscopy conducted at Argonne National Lab is used to elucidate the microstructure and crystallinity of the polymers. These data, taken together allow us to build structure property relationships describing the mobility of ions within these materials. We seek to leverage our understanding of ion transport to synthesize new single ion conducting polymers with enhanced ion transport. We hope to then explore the viability of these electrolytes with next generation electrodes such as lithium metal anodes.
Figure 1. Colors show the impact of transference number and diffusion coefficient on dendrite growth. High transference numbers result in the lowest growth. Points represent experimental data. Used with permission from Ref 4.
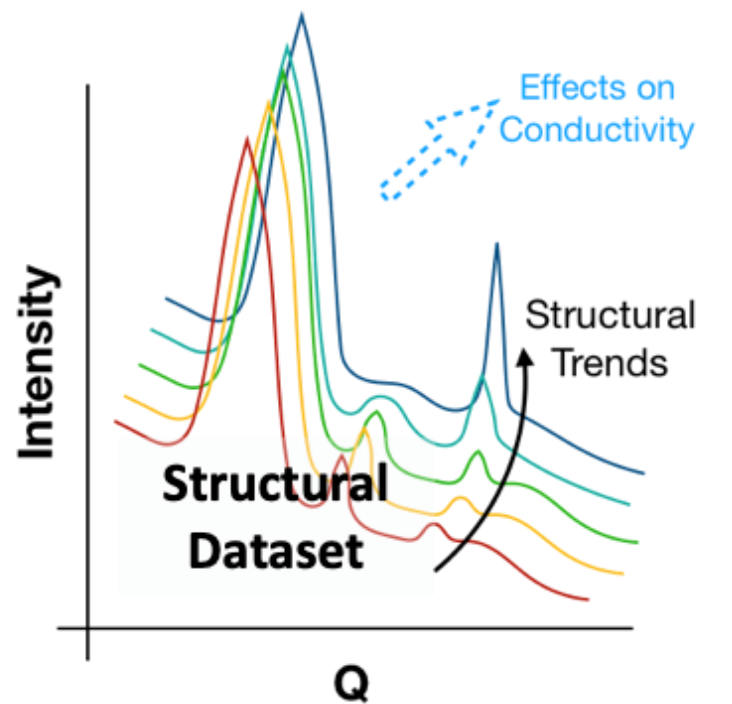
Figure 2. Structural data combined with electrochemical analysis allows the understanding of how microstructure can affect ion transport.
References:
1. Hallinan, D. T. & Balsara, N. P. Polymer Electrolytes. Annu. Rev. Mater. Res. 43, 503–525 (2013).
2. Timachova, K., Watanabe, H. & Balsara, N. P. Effect of Molecular Weight and Salt Concentration on Ion Transport and the Transference Number in Polymer Electrolytes. Macromolecules 48, 7882–7888 (2015).
3. Porcarelli, L., Shaplov, A. S., Bella, F., Nair, J. R., Mecerreyes, D., & Gerbaldi, C. Single-Ion Conducting Polymer Electrolytes for Lithium Metal Polymer Batteries that Operate at Ambient Temperature. ACS Energy Lett. 1, 678–682 (2016).
4. Li, S., Mohamed, A. I., Pande, V., Wang, H., Cuthbert, J., Pan, X., He, H., Wang, Z., Viswanathan, V., Whitacre J. F., & Matyjaszewski, K. Single-Ion Homopolymer Electrolytes with High Transference Number Prepared by Click Chemistry and Photoinduced Metal-Free Atom-Transfer Radical Polymerization. ACS Energy Lett. 3, 20–27 (2018).
5. Doyle, M., Fuller, T. F. & Newman, J. The importance of the lithium ion transference number in lithium/polymer cells. Electrochim. Acta 39, 2073–2081 (1994).
6. Elmore, C. T., Seidler, M. E., Ford, H. O., Merrill, L. C., Upadhyay, S. P., Schneider, W. F., & Schaefer, J. L. Ion Transport in Solvent-Free, Crosslinked, Single-Ion Conducting Polymer Electrolytes for Post-Lithium Ion Batteries. Batteries 4, 28 (2018).