Hydrogen Carriers
Advanced heterogeneous catalysis and electrocatalysis is the key requirement for the production and conversion of carbon-free hydrogen carriers, which is a critical step in realizing many energy conversion and storage technologies. By encompassing different experimental (synthesis and characterization) and computational techniques, the focus of our group is on understanding the electronic structures of catalyst surfaces to identify descriptors of catalytic properties. A fundamental understanding of the descriptors allows us to engineer catalytic properties and design catalysts for various reactions, such as oxygen/hydrogen electrocatalysis, CO2 reduction, methanol oxidation and CxHyOz/NOx conversion. In addition to the traditional descriptors that are mainly based on the electronic structure at catalyst surfaces, our group has also proposed an alternative path that leverages the physical chemistry of liquids, primarily the water structure at the outer-Helmholtz layer, to control the electrochemical reaction kinetics.
Additional Reading:
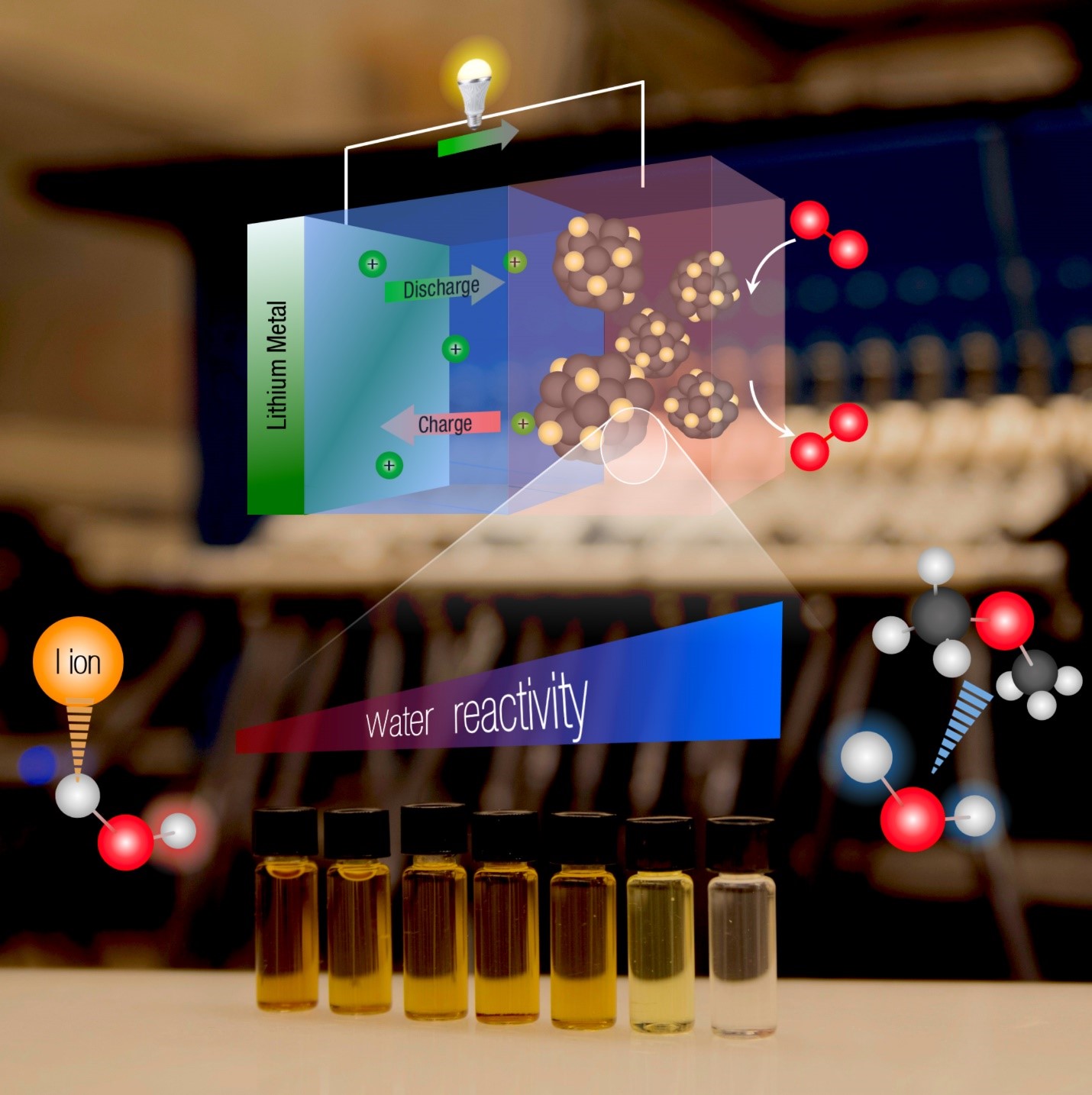
Li-ion Batteries
Li-ion batteries have been the ubiquitous energy storage technology for the past four decades, with diverse applications in consumer electronics, electric vehicles, and medical implants. In our group, we focus on understanding the reaction mechanisms of constituent chemistries in the Li-ion battery cell, including positive electrodes, negative electrodes, and electrolytes. In addition, we research electrode-electrolyte interfaces in order to develop next-generation high-energy storage batteries with higher energy density and long-term cycling stability. Our primary tools include novel in-situ and ex-situ spectroscopic techniques, density functional theory calculations, and electrochemical testing, alongside high-throughput synthesis and testing novel electrolytes.
Additional Reading:
- The Electrode-Electrolyte Interface in Li-ion Batteries: Current Understanding and New Insights
- Revealing electrolyte oxidation via carbonate dehydrogenation on Ni-based oxides in Li-ion batteries by in situ Fourier transform infrared spectroscopy
- Enhanced Cycling Performance of Ni-rich Positive Electrodes (NMC) in Li-ion Batteries by Reducing Electrolyte Free-solvent Activity
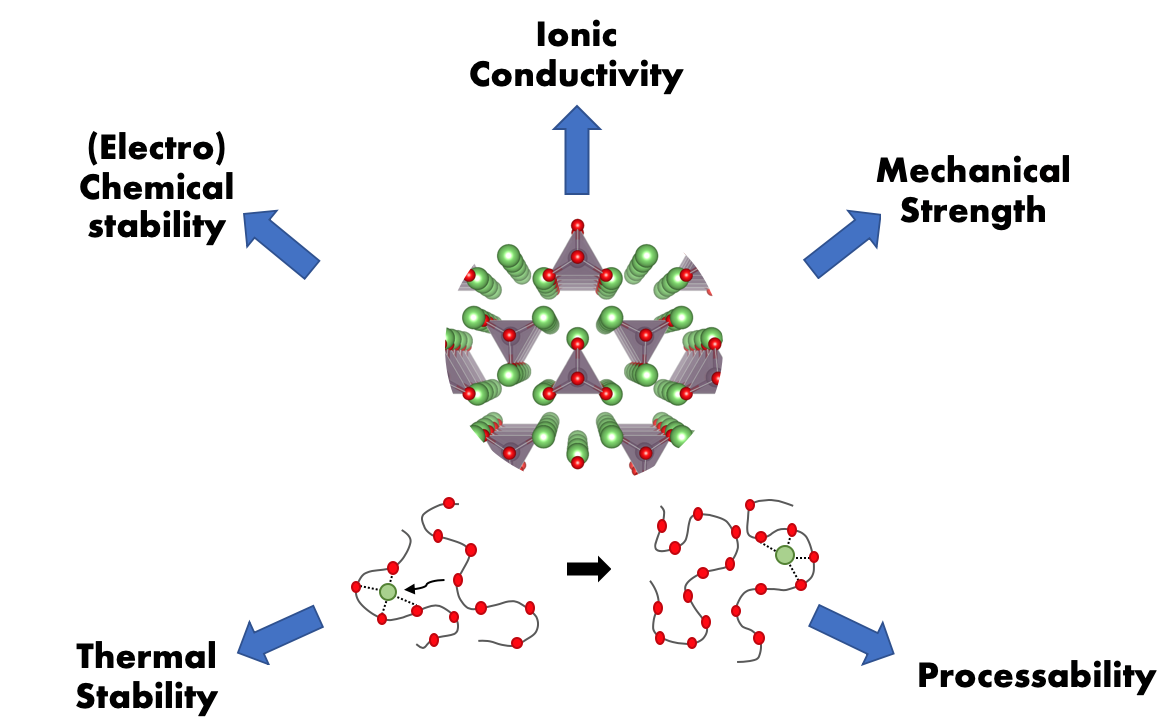
Ion-Conductors
The transport of ions through the bulk of a material is the heart of many electrochemical applications from batteries to fuel cells. Our research group studies ion conduction through a variety of media – including polymers and ceramics – with a diverse set of methods. Our goal is to understand the fundamental mechanisms of ionic conductivity and the underlying physical descriptors of ion conduction. We use high-throughput computational methods along with custom-built high-throughput equipment to screen novel materials. We hope to apply these descriptors to formulate new ion-conducting materials like ceramics and polymers that exhibit both fast ion conduction and stability against other system components. The chosen formulations are then utilized to improve the performance of different energy storage, conversion, and catalysis applications.
Additional Reading:
Beyond Li-ion
As decarbonization of the electricity grid and transportation sectors progresses, energy storage technologies with higher energy densities and lower costs than Li-ion batteries will be required. The focus of our group in research topic is to understand and develop future battery technologies. Our research ranges from performing fundamental studies, optimizing electrolyte composition, improving the performances of Li, and Na-air batteries to developing high energy density flow battery prototypes. The ultimate goal of our research is to identify strategies to propel the commercialization of batteries beyond the current limitations of Li-ion batteries to generate novel batteries for the future.
Additional Reading:
- Solvent-Dependent Oxidizing Power of LiI Redox Couples for Li-O2 Batteries
- Experimental and Computational Analysis of the Solvent-Dependent O2/Li+-O2– Redox Couple: Standard Potentials, Coupling Strength, and Implications for Lithium–Oxygen Batteries
- Influence of Edge- and Basal-plane Sites on the Vanadium Redox Kinetics for Flow Batteries